Abstract
Objectives
Relapse prevention is a major therapeutic goal in the treatment of schizophrenia. However, many patients experience multiple functional impairments and treatment resistance due to recurrence. This study was designed to investigate the follow-up of patients with using antipsychotic drugs and to compare the total treatment failure rate, withdrawal reasons, and duration period of antipsychotic drugs.
Methods
The subjects were 1963 patients who taking antipsychotic drugs under the diagnosis of schizophrenia. We selected 1836 patients using 10 antipsychotic drugs according to frequency of using. The rate of total treatment failure of them was divided into 6-month, 1-year, 2-year, 3-year, and 5-year according to the time of drug withdrawal. We compared the total treatment failure rate at 1 and 3-year between 10 antipsychotic drugs.
Results
The total treatment failure rate of clozapine was lowest compared with the other 9 antipsychotic drugs in all the surveyed periods. When evaluating actual number of subjects, olanzapine, sulpiride, risperidone, aripiprazole, amisulpride, and haloperidol were lower significantly compared with ziprasidone at 1-year in the total treatment failure rate, but there was no significant difference between them except clozapine at 3-year. The results of the analysis based on the number of prescriptions showed that the total treatment failure rate of the atypical antipsychotic drug was lower than that of the typical antipsychotic drug at 1-year, but the difference was decreased over time except quetiapine and ziprasidone.
Go to : 
References
1. Pincus HA. From PORT to policy to patient outcomes: crossing the quality chasm. Schizophr Bull. 2010; 36:109–111.

2. Huang SS, Lin CH, Loh el-W, Yang HY, Chan CH, Lan TH. Antipsychotic formulation and one-year rehospitalization of schizophrenia patients: a population-based cohort study. Psychiatr Serv. 2013; 64:1259–1262.

3. Gitlin M, Nuechterlein K, Subotnik KL, Ventura J, Mintz J, Fo-gelson DL, et al. Clinical outcome following neuroleptic discontinuation in patients with remitted recent-onset schizophrenia. Am J Psychiatry. 2001; 158:1835–1842.

4. Kane JM, Osuntokun O, Kryzhanovskaya LA, Xu W, Stauffer VL, Watson SB, et al. A 28-week, randomized, double-blind study of olanzapine versus aripiprazole in the treatment of schizophrenia. J Clin Psychiatry. 2009; 70:572–581.

5. Stroup TS, Lieberman JA, McEvoy JP, Swartz MS, Davis SM, Rosenheck RA, Hsiao JK. CATIE Investigators. Effectiveness of olanzapine, quetiapine, risperidone, and ziprasidone in patients with chronic schizophrenia following discontinuation of a previous atypical antipsychotic. Am J Psychiatry. 2006; 163:611–622.

6. Leucht S, Cipriani A, Spineli L, Mavridis D, Orey D, Richter F, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments metaanalysis. Lancet. 2013 Sep 14; 382:951–962.

7. Kahn RS, Fleischhacker WW, Boter H, Davidson M, Vergouwe Y, Keet IP, et al. Effectiveness of antipsychotic drugs in first-episode schizophrenia and schizophreniform disorder: an open randomised clinical trial. Lancet. 2008; 371:1085–1097.

8. Nyhuis AW, Faries DE, Ascher-Svanum H, Stauffer VL, Kinon BJ. Predictors of switching antipsychotic medications in the treatment of schizophrenia. BMC Psychiatry. 2010; 10:75.

9. Kane JM, Kishimoto T, Correll CU. Non-adherence to medication in patients with psychotic disorders: epidemiology, contributing factors and management strategies. World Psychiatry. 2013; 12:216–226.

10. Goff DC, Hill M, Freudenreich O. Strategies for improving treatment adherence in schizophrenia and schizoaffective disorder. J Clin Psychiatry. 2010; 71(Suppl 2):20–26.

11. Harrison G, Hopper K, Craig T, Laska E, Siegel C, Wanderling J, et al. Recovery from psychotic illness: a 15- and 25-year international follow-up study. Br J Psychiatry. 2001; 178:506–517.

12. American Psychiatric Association. The diagnostic and statistical manual of mental disorder. 4th ed., Washington DC, American Psychiatric Press;1994.
13. Ascher-Svanum H, Faries DE, Zhu B, Ernst FR, Swartz MS, Swanson JW. Medication adherence and longterm functional outcomes in the treatment of schizophrenia in usual care. J Clin Psychiatry. 2006; 67:453–460.

14. Ascher-Svanum H, Zhu B, Faries DE, Salkever D, Slade EP, Peng X, et al. The cost of relapse and the predictors of relapse in the treatment of schizophrenia. BMC Psychiatry. 2010; 10:2.

15. Peuskens J, Olivares JM, Pecenak J, Tuma I, Bij de Weg H, Eriksson L, et al. Treatment retention with risperidone long-acting injection: 24-month results from the Electronic Schizophrenia Treatment Adherence Registry (e-STAR) in six countries. Curr Med Res Opin;. 2010. 501–509.

16. Canadian Psychiatric Association. Clinical practice guidelines. Treatment of schizophrenia. Can J Psychiatry. 2005; 50(Suppl 1):7–57.
17. Geddes J1. Freemantle N, Harrison P, Bebbington P. Atypical antipsychotics in the treatment of schizophrenia: systematic overview and meta-regression analysis. BMJ. 2000; 321:1371–1376.

18. Lehman AF, Lieberman JA, Dixon LB, McGlashan TH, Miller AL, Perkins DO, et al. Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry. 2004; 161:1–56.
19. Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005; 353:1209–1223.

20. Mullins CD, Obeidat NA, Cuffel BJ, Naradzay J, Loebel AD. Risk of discontinuation of atypical antipsychotic agents in the treatment of schizophrenia. Schizophr Res. 2008; 98:8–15.

Go to : 
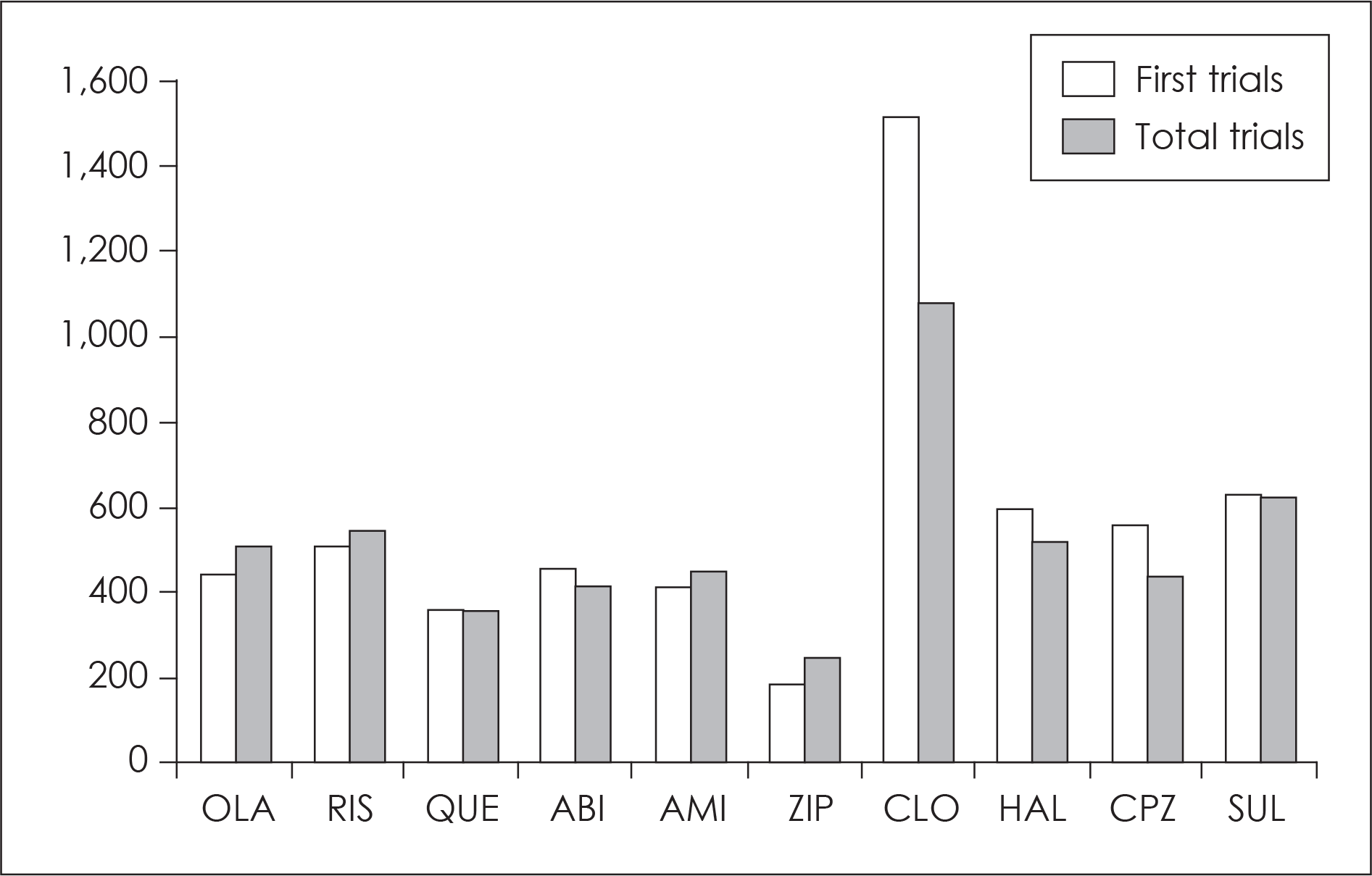 | Fig. 1.The period of continuation in antipsychotic drugs (days). OLA: olanzapine, RSP: risperidone, QUE: quetiapine, ABI: aripiprazole, ZIP: ziprasidone, CLO: clozapine, HAL: haloperidol, CPZ: chlorpramazine, SUL: sulpiride. |
Table 1.
Sociodemographic characteristics
Table 2.
Discontinuation rate according to period and reasons of discontinuation on 10 antipsychotics of trial 1 (N=1836)
Table 3.
Discontinuation rate according to period and reasons of discontinuation on 10 antipsychotic of total trials (N=5057)
Table 4.
Comparison of discontinuation rate of 10 antipsychotic agents in first trials
Table 5.
Comparison of discontinuation rate of 10 antipsychotic agents in total trials




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download