Abstract
Objectives
It has been shown that early intervention is crucial for favorable outcome in patients with schizophrenia. However, development of biomarkers for predicting prognosis of psychotic disorder still requires more research. In this study, we aimed to investigate whether baseline mismatch negativity (MMN) predict prognosis in patients with first episode psychosis (FEP).
Methods :
Twenty-four patients with FEP and matched healthy controls (HCs) were examined with MMN at baseline, and their clinical status were re-assessed after 1 year. Repeated-measures analysis of variance was performed to compare baseline MMN between the two groups. Multiple regression analysis was used to identify factors predicting prognosis in FEP patients during the followup period.
Results
MMN amplitudes at baseline were significantly reduced in patients with FEP compared to healthy controls. In the multiple regression analysis, baseline MMN amplitude significantly predicted later improvement of performances on digit span and delayed recall of California Verbal Learning Test. However, baseline MMN did not predicted improvement of clinical symptoms.
Go to : 
REFERENCES
1). McGrath J, Saha S, Welham J, El Saadi O, MacCauley C, Chant D. A systematic review of the incidence of schizophrenia: the distribution of rates and the influence of sex, urbanicity, migrant status and methodology. BMC Med. 2004; 2:1–13.

2). Edwards J, Harrigan SM, McGorry PD, Amminger PG. Duration of untreated psychosis (DUP) and outcome in schizophrenia. Psychol Med. 2002; 32:563–564.
3). Ito S, Nemoto T, Tsujino N, Ohmuro N, Matsumoto K, Matsuoka H, et al. Differential impacts of duration of untreated psychosis (DUP) on cognitive function in firstepisode schizophrenia according to mode of onset. Eur Psychiatry. 2015; 30:995–1001.

4). Yuksel C, McCarthy J, Shinn A, Pfaff DL, Baker JT, Heckers S, et al. Gray matter volume in schizophrenia and bipolar disorder with psychotic features. Schizophr Res. 2012; 138:177–182.
5). Horan WP, Green MF, DeGroot M, Fiske A, Hellemann G, Kee K, et al. Social cognition in schizophrenia, Part 2: 12-month stability and prediction of functional outcome in firstepisode patients. Schizophr Bull. 2012; 38:865–872.

6). Huang J, Tan SP, Walsh SC, Spriggens LK, Neumann DL, Shum DH, et al. Working memory dysfunctions predict social problem solving skills in schizophrenia. Psychiatry Res. 2014; 220:96–101.

7). Prouteau A, Verdoux H, Briand C, Lesage A, Lalonde P, Nicole L, et al. The crucial role of sustained attention in community functioning in outpatients with schizophrenia. Psychiatry Res. 2004; 129:171–177.

8). Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the “right stuff”? Schizophr Bull. 2000; 26:119–136.

9). Dell'Osso B, Glick ID, Baldwin DS, Altamura AC. Can longterm outcomes be improved by shortening the duration of untreated illness in psychiatric disorders? A conceptual framework. Psychopa-thology. 2013; 46:14–21.
10). van der Gaag M, Smit F, Bechdolf A, French P, Linszen DH, Yung AR, et al. Preventing a first episode of psychosis: metaanalysis of randomized controlled prevention trials of 12 month and longer-term followups. Schizophr Res. 2013; 149:56–62.
11). Fond G, d'Albis MA, Jamain S, Tamouza R, Arango C, Fleischhacker WW, et al. The promise of biological markers for treatment re-sponse in firstepisode psychosis: a systematic review. Schizophr Bull. 2015; 41:559–573.

12). Dancey JE, Dobbin KK, Groshen S, Jessup JM, Hruszkewycz AH, Koehler M, et al. Guidelines for the development and incorporation of biomarker studies in early clinical trials of novel agents. Clin Cancer Res. 2010; 16:1745–1755.

13). Yener GG, Basar E. Brain oscillations as biomarkers in neuropsy-chiatric disorders: following an interactive panel discussion and synopsis. Suppl Clin Neurophysiol. 2013; 62:343–363.
14). Bramon E, Croft RJ, McDonald C, Virdi GK, Gruzelier JG, Bal-deweg T, et al. Mismatch negativity in schizophrenia: a family study. Schizophr Res. 2004; 67:1–10.

15). Shin KS, Kim JS, Kim SN, Koh Y, Jang JH, An SK, et al. Aberrant auditory processing in schizophrenia and in subjects at ultra-high-risk for psychosis. Schizophr Bull. 2012; 38:1258–1267.

16). Light GA, Williams LE, Minow F, Sprock J, Rissling A, Sharp R, et al. Electroencephalography (EEG) and event-related potentials (ERPs) with human participants. Curr Protoc Neurosci. 2010; 25:21–24.

17). Rydkjaer J, Mollegaard Jepsen Jr, Pagsberg AK, Fagerlund B, Gl-enthoj BY, Oranje B. Mismatch negativity and P3a amplitude in young adolescents with firstepisode psychosis: a comparison with ADHD. Psychol Med. 2017; 47:377–388.
18). Sumiyoshi T, Miyanishi T, Seo , Higuchi Y. Electrophysiological and neuropsychological predictors of conversion to schizophrenia in at-risk subjects. Front Behav Neurosci. 2013; 7:148.

19). Naatanen R, Tervaniemi M, Sussman E, Paavilainen P, Winkler I. “Primitive intelligence” in the auditory cortex. Trends Neurosci. 2001; 24:283–288.
20). Javitt DC, Doneshka P, Zylberman I, Ritter W, Vaughan HG Jr.Impairment of early cortical processing in schizophrenia: an event-related potential confirmation study. Biol Psychiatry. 1993; 33:513–519.

21). Michie PT, Budd TW, Todd J, Rock D, Wichmann H, Box J, et al. Duration and frequency mismatch negativity in schizophrenia. Clin Neurophysiol. 2000; 111:1054–1065.

22). Sevik AE, Anil Yagcioglu AE, Yagcioglu S, Karahan S, Gurses N, Yildiz M. Neuropsychological performance and auditory event related potentials in schizophrenia patients and their siblings: a family study. Schizophr Res. 2011; 130:195–202.
Go to : 
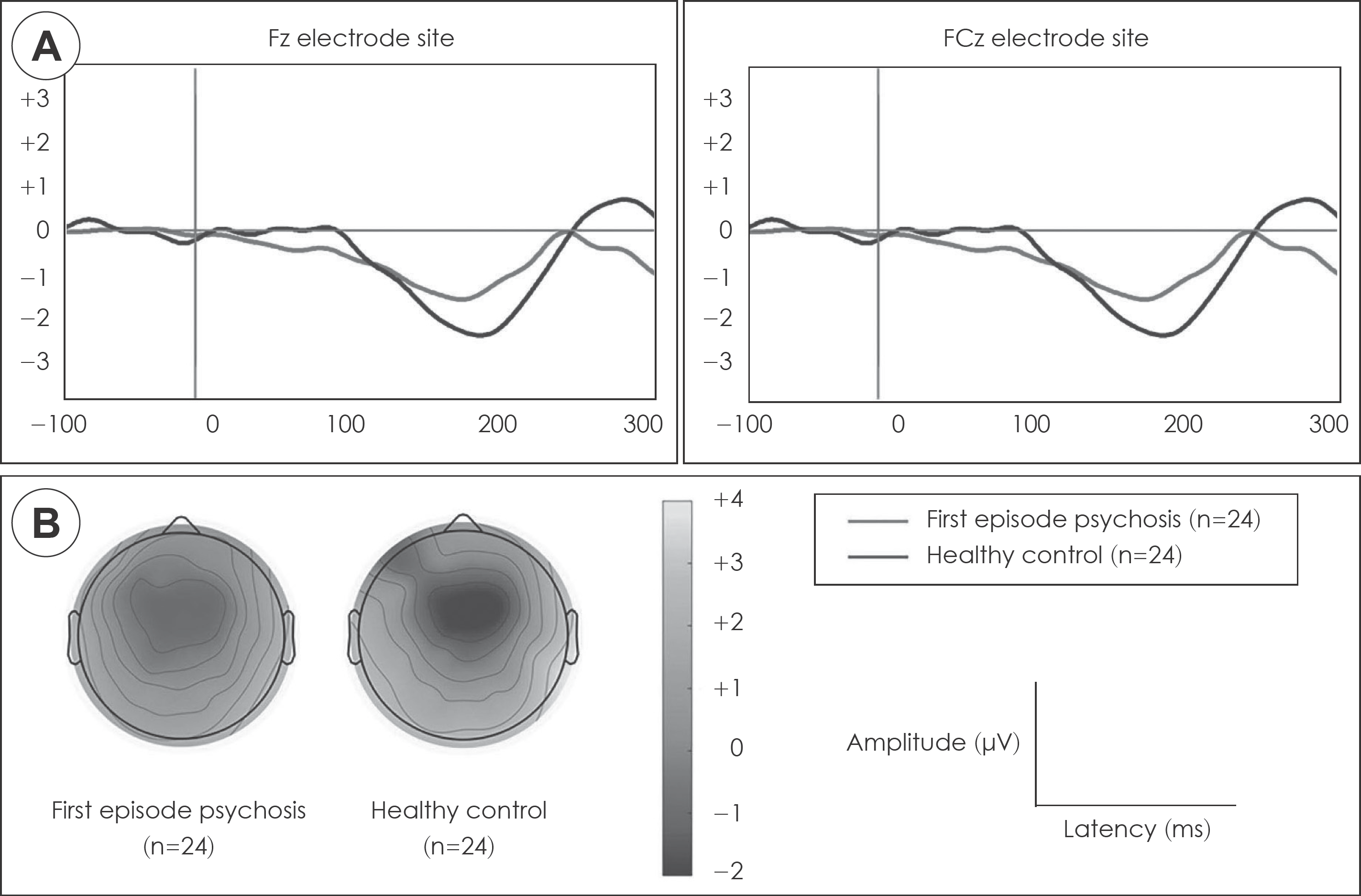 | Fig. 1.A : The grand averaged Mismatch negativity waveforms at Fz and FCz electrode sites in patients with FEP and HC. B : Two-dimensional topographic maps of MMN across FEP and HC groups. Color bar with numbers indicates MMN amplitude (μV). FEP : first episode psychosis, HC : healthy controls. |
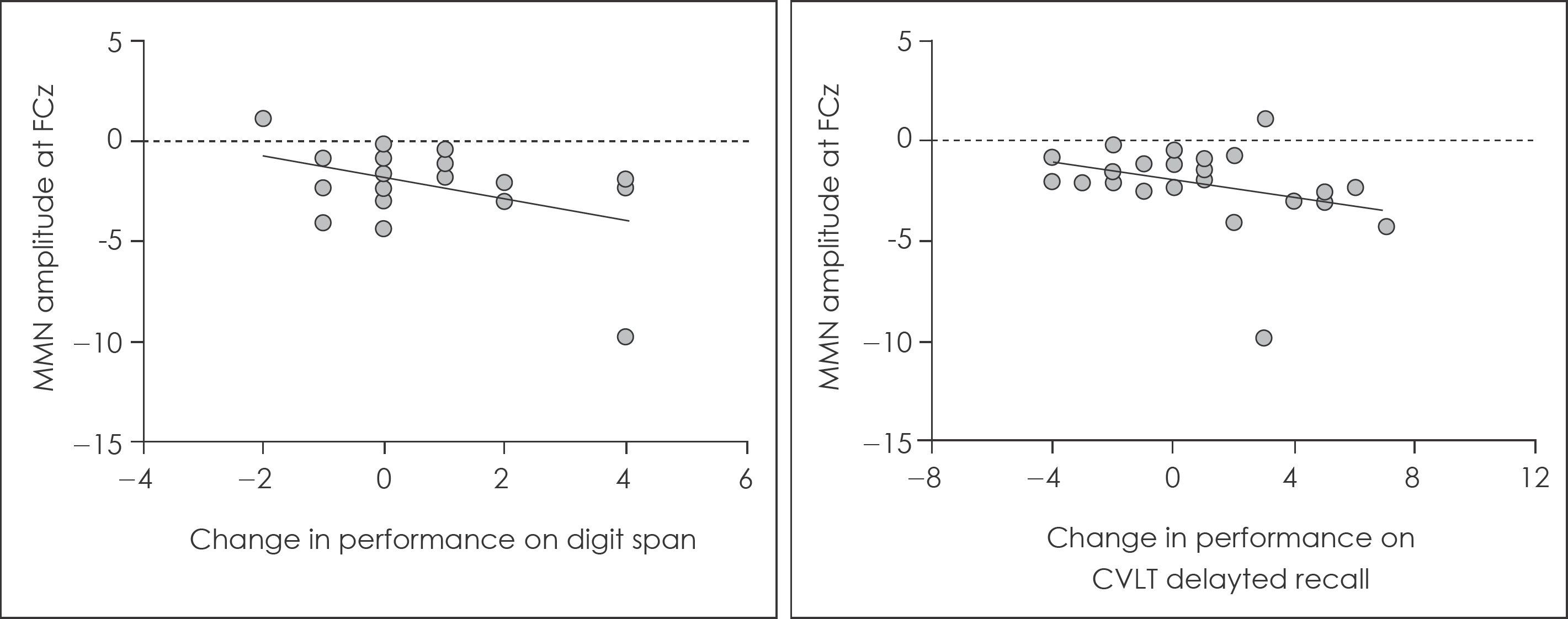 | Fig. 2.Association between baseline MMN amplitude at FCz electrode site and improvement in performances of neurocognitive function tests. CVLT : California Verbal Learning Test. |
Table 1.
Demographic, clinical, and neurocognitive characteristics of the patients with FEP and HCs at baseline
|
FEP (N=24) |
HC (N=24) |
Statistical analysis∗ | ||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | χ2 or T | p | |
| Sex (Male/Female) | 10/14 | 9/15 | 0.087 | 0.500 | ||
| Handedness (Right/Left) | 22/2 | 22/2 | - | 0.696 | ||
| Age (years) | 22.4 | 1.0 | 23.5 | 4.0 | -0.813 | 0.420 |
| IQ | 98.0 | 2.8 | 118.2 | 13.4 | -5.160 | <0.001‡ |
| Education (years) | 13.3 | 0.4 | 14.6 | 1.6 | -2.488 | 0.017† |
| DUP (months) | 5.7 | 0.8 | - | - | - | - |
| PANSS | ||||||
| Total | 69.1 | 14.0 | - | - | - | - |
| Positive symptoms | 17.3 | 4.6 | - | - | - | - |
| Negative symptoms | 17.3 | 4.6 | - | - | - | - |
| General symptoms | 34.5 | 7.1 | - | - | - | - |
| NCFT measures | ||||||
| Working memory | ||||||
| Digit span | 10.6 | 1.8 | 12.8 | 2.2 | -3.844 | <0.001‡ |
| Attention | ||||||
| Trail Making Test, part A | 29.0 | 9.1 | 22.3 | 7.4 | 2.8 | 0.008† |
| Verbal memory | ||||||
| CVLT, immediate recall | 8.2 | 3.2 | 12.9 | 2.2 | -5.990 | <0.001‡ |
| CVLT, delayed recall | 8.6 | 3 | 13.3 | 2.2 | -6.092 | <0.001‡ |
Table 2.
Follow-up characteristics and change in symptoms as well as neurocognitive functioning during the 1 year of followup period of the patients with FEP
|
FEP (N=24) |
||
|---|---|---|
| Mean | SD | |
| Antipsychotics dose∗ | 21.0 4 | 47.56 |
| Use of medication† | ||
| Antipsychotics | 24 (100) | |
| Antidepressants | 10 (41.7) | |
| Mood stabilizers | 4 (16.7) | |
| Anxiolytics | 19 (79.2) | |
| Change in PANSS‡ | ||
| Total | 22.4 | 17.6 |
| Positive symptoms | 7.1 | 5.0 |
| Negative symptoms | 4.5 | 5.9 |
| General syptoms | 10.8 | 9.3 |
| Changes in NCFT‡ | ||
| Working memory | ||
| Digit span | 0.6 | 1.6 |
| Attention | ||
| Trail making test, part A | 3.7 | 9.3 |
| Verbal memory | ||
| CVLT, immediate recall | 0.7 | 3.0 |
| CVLT, delayed recall | 0.9 | 3.1 |
Table 3.
Means and SD of MMN peak amplitudes and latencies in patients with FEP and HCs at surface electrodes
| Electrode sites |
FEP (N=24) |
HC (N=24) |
Statistical Analysis∗ |
|||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | T | p | |
| Amplitude (μV) | ||||||
| F3 | -1.8 | 1.2 | -2.3 | 1.3 | 1.207 | 0.234 |
| Fz | -1.8 | 1.0 | -2.8 | 1.3 | 2.770 | 0.008† |
| F4 | -1.9 | 1.0 | -2.9 | 1.2 | 3.211 | 0.002‡ |
| FC3 | -1.7 | 0.9 | -1.9 | 1.1 | 0.948 | 0.348 |
| FCz | -2.1 | 2.0 | -2.8 | 1.2 | 1.529 | 0.133 |
| FC4 | -1.8 | 1.0 | -2.7 | 1.1 | 3.162 | 0.003‡ |
| Latency (ms) | ||||||
| F3 | 181.7 | 31.7 | 183.3 | 25.9 | -0.200 | 0.843 |
| Fz | 179.2 | 35.6 | 182.3 | 27.1 | -0.351 | 0.727 |
| F4 | 179.2 | 37.9 | 184.7 | 28.2 | -0.566 | 0.574 |
| FC3 | 212.9 | 201.3 | 176.0 | 32.9 | 0.884 | 0.381 |
| FCz | 175.5 | 29.9 | 184.5 | 25.4 | -1.128 | 0.265 |
| FC4 | 179.3 | 38.3 | 174.9 | 27.5 | 0.485 | 0.630 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download