Abstract
Objectives
This study investigated the prescribing patterns of atypical antipsychotics for the various psychiatric disorders in the psychiatric ward of a University hospital.
Methods
We reviewed the medical records of patients who were discharged from an open psychiatric ward from May, 2003 through April, 2014. The association between psychiatric disorders and prescription pattern of atypical antipsychotics was analyzed.
Results
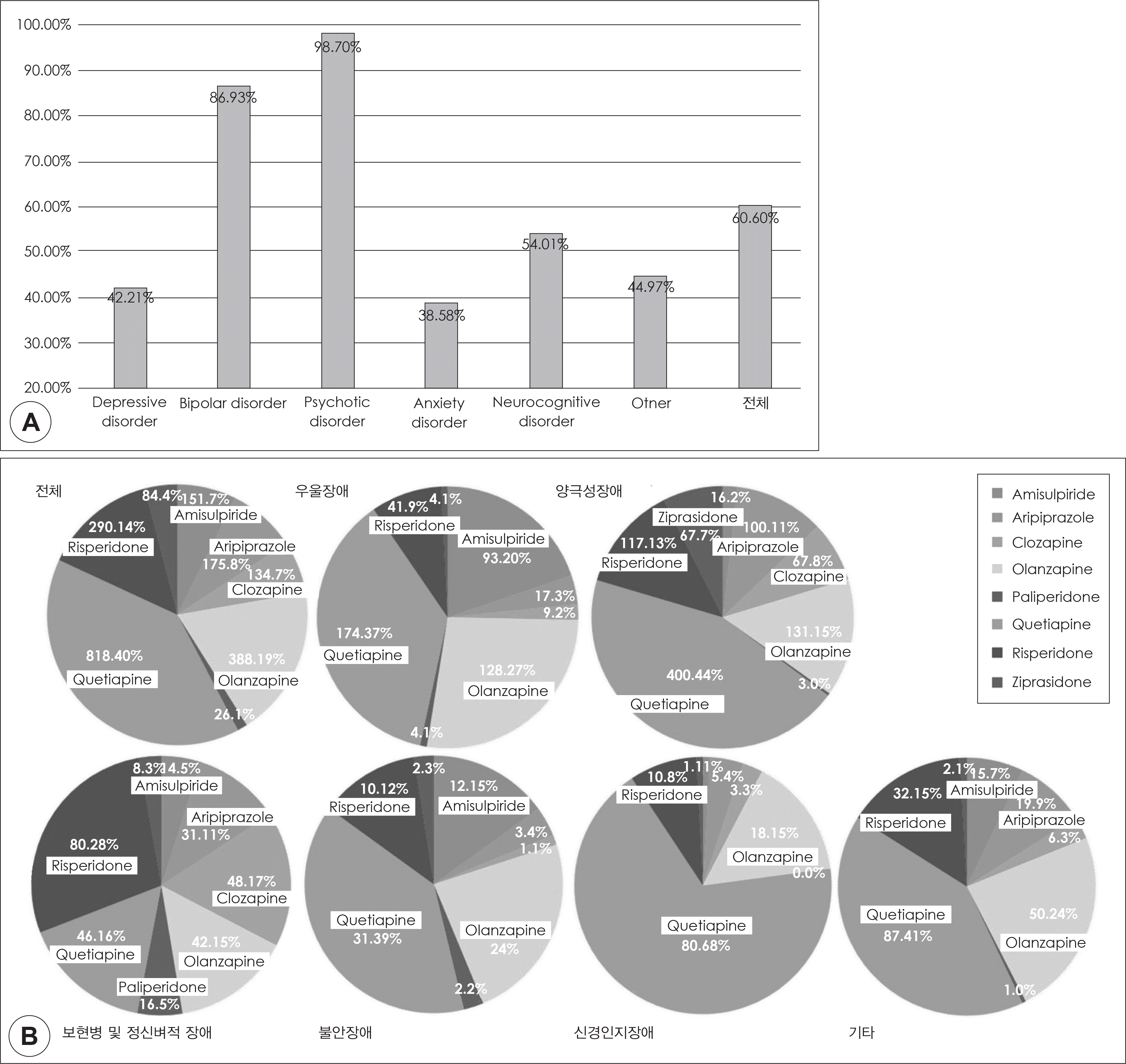
: The study included 3091 patients' prescription of psychiatric medication. 60% of prescription included antipsychotics; quetiapine was the most frequently prescribed antipsychotics, but the average dosage was the lowest among all the atypical antipsychotics. According to the diagnoses, prescription rates and dosage of antipsychotics were different. Prescription rates of antipsychotics were the lowest in patients with anxiety disorders, and the mean dosage were the lowest in those with delirium, dementia, and amnestic and other cognitive disorders.
Conclusion
This observational study shows prescription patterns of atypical antipsychotics for the treatment of psychiatric disorders in a University hospital; atypical antipsychotics were widely used for the treatment of the various disorders, and there were differences in prescription patterns for each disorders. The results of this study may be used to identify the proper atypical antipsychotics effective on certain psychiatric disorders and to propose expanding the indications of each atypical antipsychotics in the future.
Go to : 
References
1. Olfson M, Blanco C, Liu SM, Wang S, Correll CU. National trends in the office-based treatment of children, adolescents, and adults with antipsychotics. Archives of General Psychiatry. 2012; 69:1247–1256.

2. Crystal S, Olfson M, Huang C, Pincus H, Gerhard T. Broadened use of atypical antipsychotics: safety, effectiveness, and policy challenges. Health Affairs. 2009; 28:w770–w781.

3. Rothbard AB, Kuno E, Foley K. Trends in the rate and type of antipsychotic medications prescribed to persons with schizophrenia. Schizophrenia Bulletin. 2003; 29:531–540.

4. Diatta T, Blazejewski S, Portier A, Lignot S, Quesnot A, Moore N, Fourrier-Réglat A. Patterns and frequency of atypical antipsychotic prescribing in psychiatric medical centers: a cross-sectional national survey. Fundamental & Clinical Pharmacology. 2007; 21:371–378.

7. Dolder CR, Lacro JP, Dunn LB, Jeste DV. Antipsychotic medication adherence: is there a difference between typical and atypical agents? American Journal of Psychiatry. 2002; 159:103–108.

8. Maher AR, Maglione M, Bagley S, Suttorp M, Hu JH, Ewing B, Shekelle PG. Efficacy and comparative effectiveness of atypical antipsychotic medications for off-label uses in adults: a systematic review and metaanalysis. JAMA. 2011; 306:1359–1369.
9. Maher AR, Theodore G. Summary of the comparative effectiveness review on off-label use of atypical antipsychotics. Journal of Managed Care Pharmacy. 2012; 18:1–20.

10. Byun SJ, Kim ET. Changes in Psychotropic prescription patterns in patients admitted to an open psychiatric ward: 11-year comparison in a University Hospital. Korean Journal of Biological Psychiatry. 2015; 22:4.
11. Kwon JS, Kim ET, Ha TH, Roh KS, Choi JS, Kim YS. Drug prescribing patterns of outpatients with schizophrenia in a university hospital. Journal of Korean Neuropsychiatric Association. 2003; 42:683–690.
12. Gardner DM, Murphy AL, O'Donnell H, Centorrino F, Baldessa-rini RJ. International consensus study of antipsychotic dosing. American Journal of Psychiatry. 2010; 167:686–693.

13. Bret MC, Bret P, Pariente A, Fourier-Reglat A. The use of atypical antipsychotics in French psychiatric hospitals. Pharmacy World & Science. 2007; 29:551–556.

14. Migler BM, Warawa EJ, Malick JB. Seroquel: behavioral effects in conventional and novel tests for atypical antipsychotic drug. Psychopharmacology. 1993; 112:299–307.

15. McManus DQ. Quetiapine, a novel antipsychotic: experience in elderly patients with psychotic disorders. The Journal of Clinical Psychiatry. 1999; 60:292–298.
16. McDonagh M, Peterson K, Carson S, Fu R, Thakurta S. Drug class review: atypical antipsychotic drugs;2010.
17. McEvoy JP, Lieberman JA, Perkins DO, Hamer RM, Gu H, Lazarus A, Strakowski SD. Efficacy and tolerability of olanzapine, quetiapine, and risperidone in the treatment of early psychosis: a randomized, double-blind 52-week comparison. American Journal of Psychiatry. 2007; 164:1050–1060.

18. Miller DD, Caroff SN, Davis SM, Rosenheck RA, McEvoy JP, Saltz BL, et al. Extrapyramidal side-effects of antipsychotics in a randomised trial. Br J Psychiatry. 2008; 193:279–288.
19. Keck PE, Orsulak PJ, Cutler AJ, Sanchez R, Torbeyns A, Marcus RN, et al. Aripiprazole monotherapy in the treatment of acute bipolar I mania: a randomized, double-blind, placebo- and lithium-controlled study. J Affect Disord. 2009; 112:36–49.

20. Young AH, Oren DA, Lowy A, McQuade RD, Marcus RN, Carson WH, et al. Aripiprazole monotherapy in acute mania: 12-week randomised placebo- and haloperidol-controlled study. Br J Psychiatry. 2009; 194:40–48.

21. Scherk H, Pajonk FG, Leucht S. Second-generation antipsychotic agents in the treatment of acute mania: a systematic review and metaanalysis of randomized controlled trials. Arch Gen Psychiatry. 2007; 64:442–455.
22. Smith LA, Cornelius V, Warnock A, Tacchi MJ, Taylor D. Pharmacological interventions for acute bipolar mania: a systematic review of randomized placebocontrolled trials. Bipolar Disorders. 2007; 9:551–560.

23. Krakowski MI, Czobor P, Citrome L, Bark N, Cooper TB. Atypical antipsychotic agents in the treatment of violent patients with schizophrenia and schizoaffective disorder. Arch Gen Psychiatry. 2006; 63:622–629.

24. Volavka J, Czobor P, Nolan K, Sheitman B, Lindenmayer JP, Citrome L, et al. Overt Aggression and Psychotic Symptoms in Patients With Schizophrenia Treated With Clozapine, Olanzapine, Risperidone, or Haloperidol. J Clin Psychopharmacol. 2004; 24:225–228.

25. Snaterse M, Welch R. A retrospective, naturalistic review comparing clinical outcomes of in-hospital treatment with risperidone and olanzapine. Clinical Drug Investigation. 2000; 20:159–164.

26. Taylor DM, Wright T, Libretto SE, Risperidone Olanzapine Drug Outcomes Studies in Schizophrenia UKIG. Risperidone compared with olanzapine in a naturalistic clinical study: a cost analysis. J Clin Psychiatry. 2003; 64:589–597.
27. Kasper S, Rosillon D, Duchesne I. Risperidone Olanzapine Drug Outcomes studies in Schizophrenia (RODOS): Efficacy and tolerability results of an international naturalistic study. Int Clin Psychopharmacol. 2001; 16:179–187.

28. Lucey JV, Libretto SE. Risperidone compared with olanzapine in a naturalistic clinical study in Ireland: a cost analysis. Ir J Med Sci. 2003; 172:195–201.

29. Danion JM, Rein W, Fleurot O, Amisulpride Study Group. Improvement of schizophrenic patients with primary negative symptoms treated with amisulpride. American Journal of Psychiatry. 1999; 156:610–616.
30. Lecrubier Y, Boyer P, Turjanski S, Rein W, Amisulpride Study Group. Amisulpride versus imipramine and placebo in dysthymia and major depression. Journal of Affective Disorders. 1997; 43:95–103.
31. Lecrubier Y, Puech AJ, Aubin F, Boyer P. Improvement by amisulpride of the negative syndrome in nonpsychotic subjects: A preliminary study. Psychiatrie & Psychobiologie. 1988; 3:329–333.
32. Maglione M, Maher AR, Hu J, Wang Z, Shanman R, Shekelle PG, Motala A. Off-label use of atypical antipsychotics: an update;2011.
33. Maher AR, Theodore G. Summary of the comparative effectiveness review on off-label use of atypical antipsychotics. Journal of Managed Care Pharmacy;. 2012. 18.

34. Sultzer DL, Davis SM, Tariot PN, Dagerman KS, Lebowitz BD, Lyketsos CG, Catie-AD Study Group. Clinical symptom responses to atypical antipsychotic medications in Alzheimer's disease: phase 1 outcomes from the CATIE-AD effectiveness trial. American Journal of Psychiatry. 2008; 165:844–854.
35. Maina G, Pessina E, Albert U, Bogetto F. 8-week, single-blind, randomized trial comparing risperidone versus olanzapine augmentation of serotonin reuptake inhibitors in treatment-resistant obsessive-compulsive disorder. European Neuropsychopharmacology. 2008; 18:364–372.

36. Matsunaga H, Nagata T, Hayashida K. A longterm trial of the effectiveness and safety of atypical antipsychotic agents in augmenting SSRI-refractory obsessive-compulsive disorder. The Journal of Clinical Psychiatry. 2009; 70:863–868.

37. Rapoport M, Mamdani M, Shulman KI, Herrmann N, Rochon PA. Antipsychotic use in the elderly: shifting trends and increasing costs. International Journal of Geriatric Psychiatry. 2005; 20:749–753.

38. Verdoux H, Tournier M, Begaud B. Antipsychotic prescribing trends: a review of pharmaco-epidemiological studies. Acta Psychiatrica Scandinavica. 2010; 121:4–10.

39. Jeste DV, Blazer D, Casey D, Meeks T, Salzman C, Schneider L, Yaffe K. ACNP White Paper: update on use of antipsychotic drugs in elderly persons with dementia. Neuropsychopharmacology. 2008; 33:957–970.

40. Schneider LS, Dagerman KS, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: metaanalysis of randomized placebocontrolled trials. JAMA. 2005; 294:1934–1943.
Go to : 
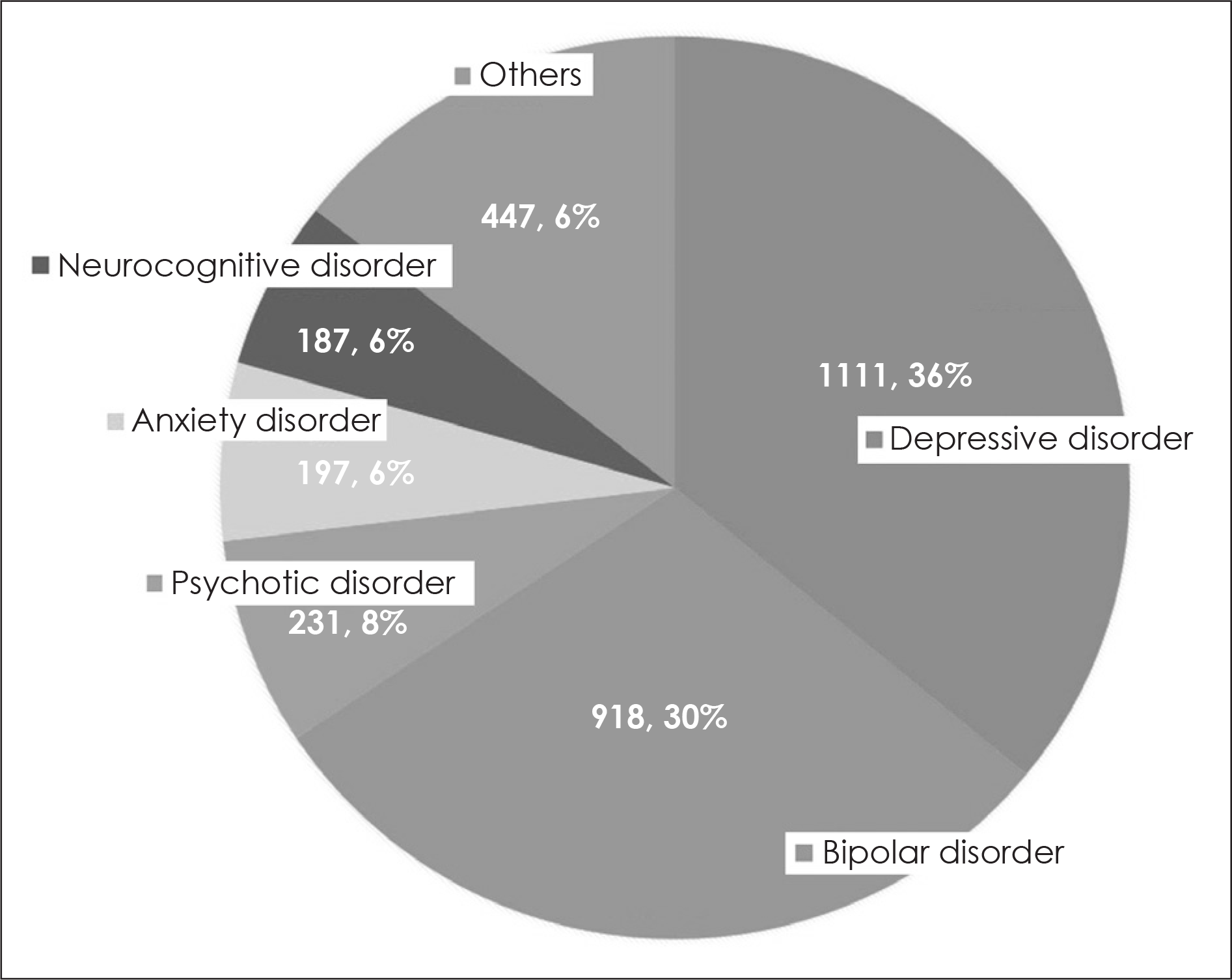 | Fig. 1.진단 분포: 우울장애 1111명(36%), 양극성장애 918명(30%), 조현 병 및 정신병적 장애 231명(8%), 불안장애 197명(6%), 신경인지장애 187 명(6%), 기타 진단군 447명(14%). |
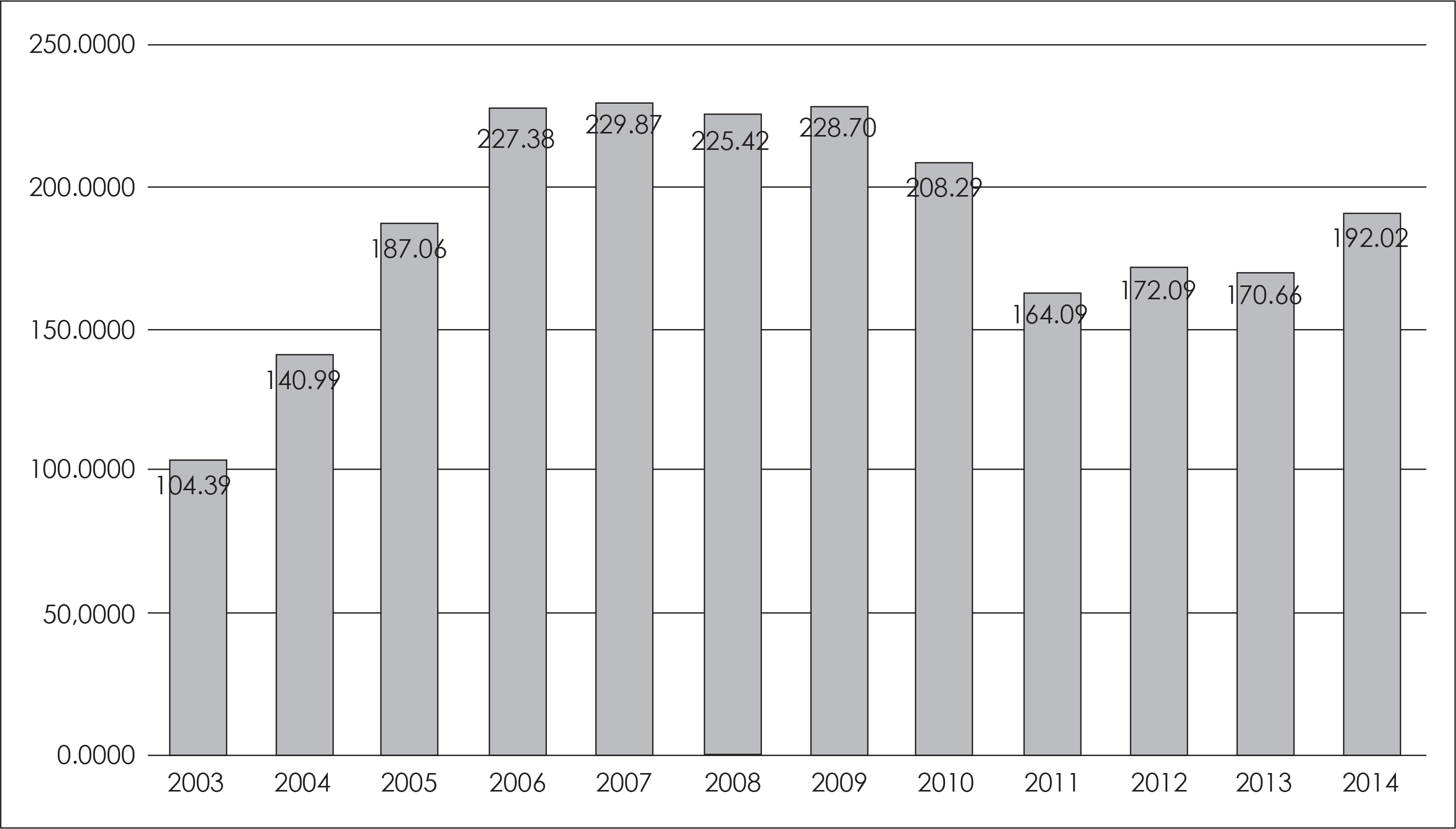 | Fig. 3.연도별 1인당 평균 항정신병제 처 방 용량(chloropromazine 등가용량). ∗: 평균 처방 용량 계산에는 항정신병 제를 처방받은 환자만을 포함시킴. |
 | Fig. 4.진단별 1인당 평균 항정신병제 처방 용량(chloropromazine 등가용 량). ∗: 평균 처방 용량 계산에는 항정 신병제를 처방받은 환자만을 포함시킴. |
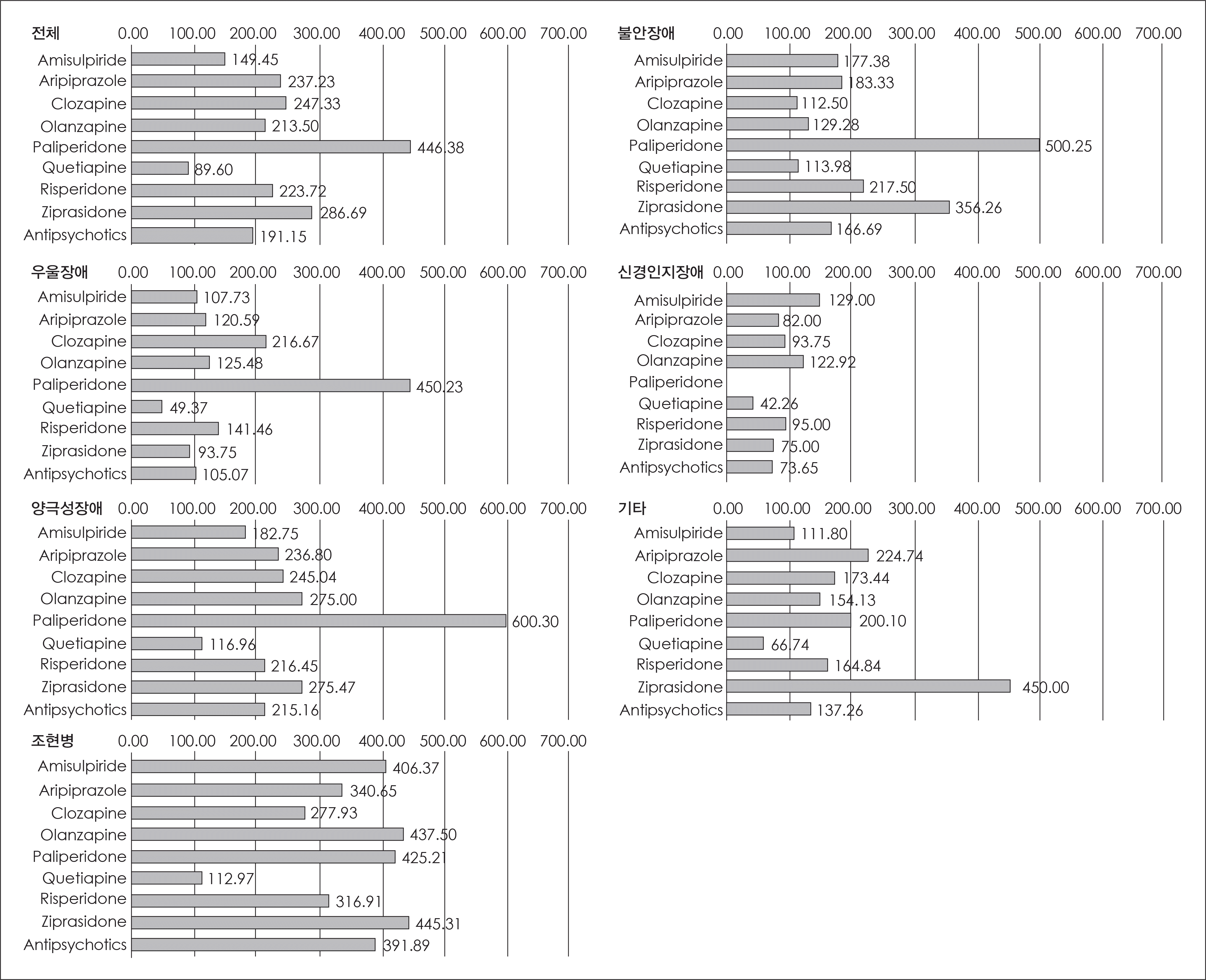 | Fig. 6.진단별 1인당 항정신병제 평균 처방 용량(Chloropromazine 등가용량). ∗: 평균 처방 용량 계산에는 항정신병제를 처방받은 환자만을 포함시킴. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download