Abstract
Objectives
It has been constantly reported that mismatch negativity (MMN) is impaired in patients with schizophrenia. However, the mechanism which relates impaired MMN and schizophrenia is not clear yet. The aim of this study is to investigate the association between MMN and clinical variables including functional status in patients with schizophrenia.
Methods
The present study assessed MMN using passive auditory oddball task in 26 patients with schizophrenia and 48 healthy controls. Repeated measures Analysis of Variance with age as a covariate was carried out for comparing peak amplitude and latency of MMN at 8 central line electrodes (FPz, Fz, FCz, Cz, CPz, Pz, POz, Oz) across groups. Pearson's correlation was performed to reveal the relationship between MMN and clinical variables including neurocognitive test results and the Global Assessment of Functioning score.
REFERENCES
1). McGrath J, Saha S, Welham J, El Saadi O, MacCauley C, Chant D. A systematic review of the incidence of schizophrenia: the distribution of rates and the influence of sex, urbanicity, migrant status and methodology. BMC Med. 2004; 2:1–13.

2). Goldman-Rakic PS. Working memory dysfunction in schizophrenia. J Neuropsychiatry Clin Neurosci. 1994; 6:348–357.
3). Green MF, Nuechterlein KH, Gaier DJ. Sustained and selective attention in schizophrenia. Prog Exp Pers Psychopathol Res. 1992; 15:290–313.
4). Schaefer J, Giangrande E, Weinberger DR, Dickinson D. The global cognitive impairment in schizophrenia: Consistent over decades and around the world. Schizophr Res 2013 in press.
5). Velligan DI, Bow-Thomas CC, Mahurin RK, Miller AL, Halguns-eth LC. Do specific neurocognitive deficits predict specific domains of community function in schizophrenia? J Nerv Ment Dis. 2000; 188:518–524.

6). Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the “right stuff”? Schizophr Bull. 2000; 26:119–136.

7). Shutara Y, Koga Y, Fujita K, Takeuchi H, Mochida M, Takemasa K. An event-related potential study on the impairment of automatic processing of auditory input in schizophrenia. Brain Topogr. 1996; 8:285–289.

8). Umbricht D, Krljes S. Mismatch negativity in schizophrenia: a metaanalysis. Schizophr Res. 2005; 76:1–23.

9). Jahshan C, Cadenhead KS, Rissling AJ, Kirihara K, Braff DL, Light GA. Automatic sensory information processing abnormalities across the illness course of schizophrenia. Psychol Med. 2012; 42:85–97.

10). Javitt DC, Doneshka P, Grochowski S, Ritter W. Impaired mismatch negativity generation reflects widespread dysfunction of working memory in schizophrenia. Arch Gen Psychiatry. 1995; 52:550–558.

11). Rissling AJ, Makeig S, Braff DL, Light GA. Neurophysiologic markers of abnormal brain activity in schizophrenia. Curr Psychiatry Rep. 2010; 12:572–578.

12). Naatanen R, Tervaniemi M, Sussman E, Paavilainen P, Winkler I. “Primitive intelligence” in the auditory cortex. Trends Neurosci. 2001; 24:283–288.
13). Naatanen R, Alho K. Mismatch negativity: the measure for central sound representation accuracy. Audiol Neurootol. 1997; 2:341–353.
14). Catts SV, Shelley AM, Ward PB, Liebert B, McConaghy N, Andrews S, et al. Brain potential evidence for an auditory sensory memory deficit in schizophrenia. Am J Psychiatry. 1995; 152:213–219.
15). Javitt DC, Shelley AM, Silipo G, Lieberman JA. Deficits in auditory and visual context-dependent processing in schizophrenia: defining the pattern. Arch Gen Psychiatry. 2000; 57:1131–1137.
16). Michie PT, Budd TW, Todd J, Rock D, Wichmann H, Box J, et al. Duration and frequency mismatch negativity in schizophrenia. Clin Neurophysiol. 2000; 111:1054–1065.

17). Salisbury DF, Shenton ME, Griggs CB, Bonner-Jackson A, McCa-rley RW. Mismatch negativity in chronic schizophrenia and firstepisode schizophrenia. Arch Gen Psychiatry. 2002; 59:686–694.

18). Atkinson RJ, Michie PT, Schall U. Duration mismatch negativity and P3a in firstepisode psychosis and individuals at ultra-high risk of psychosis. Biol Psychiatry. 2012; 71:98–104.

19). Shin KS, Kim JS, Kim SN, Koh Y, Jang JH, An SK, et al. Aberrant auditory processing in schizophrenia and in subjects at ultra-high-risk for psychosis. Schizophr Bull. 2012; 38:1258–1267.

20). Toyomaki A, Kusumi I, Matsuyama T, Kako Y, Ito K, Koyama T. Tone duration mismatch negativity deficits predict impairment of executive function in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2008; 32:95–99.

21). Wynn JK, Sugar C, Horan WP, Kern R, Green MF. Mismatch negativity, social cognition, and functioning in schizophrenia patients. Biol Psychiatry. 2010; 67:940–947.

22). Sevik AE, Anil Yagcioglu AE, Yagcioglu S, Karahan S, Gurses N, Yildiz M. Neuropsychological performance and auditory event related potentials in schizophrenia patients and their siblings: a family study. Schizophr Res. 2011; 130:195–202.
23). Michael BS, Robert L, Gibbon M, Williams , Janet BW. Structured Clinical Interview for DSM-IV Axis I Disorders. Washington DC. 1996.
24). American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed.Washington DC;1994.
25). Kim MA, Kim SN, Lee S, Byun MS, Shin KS, Park HY, et al. Impaired mismatch negativity is associated with current functional status rather than genetic vulnerability to schizophrenia Psychiatry Res: Neuroimaging 2013 in revision.
26). Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987; 13:261–276.

27). Overall JE, Gorham , Donald R. The Brief Psychiatric Rating Scale. Psychological Reports. 1967; 11:213–218.

28). Yeom TH, Park YS, Oh KJ, Kim JG, Lee YH. K-WAIS manual. Seoul: Korea Guidance;1992.
29). Naatanen R. The role of attention in auditory information processing as revealed by event-related potentials and other brain measures of cognitive function. Behavioral and Brain Science. 1990; 13:201–288.
30). Aylward E, Walker E, Bettes B. Intelligence in schizophrenia: metaanalysis of the research. Schizophr Bull. 1984; 10:430–459.

31). Allen DN, Barchard KA. Identification of a social cognition construct for the WAIS-III. Appl Neuropsychol. 2009; 16:262–274.

32). Hermens DF, Ward PB, Hodge MA, Kaur M, Naismith SL, Hickie IB. Impaired MMN/P3a complex in firstepisode psychosis: cognitive and psychosocial associations. Prog Neuropsychopharmacol Biol Psychiatry. 2010; 34:822–829.

33). Kawakubo Y, Kasai K. Support for an association between mismatch negativity and social functioning in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2006; 30:1367–1368.

34). Kawakubo Y, Kamio S, Nose T, Iwanami A, Nakagome K, Fukuda M, et al. Phonetic mismatch negativity predicts social skills acquisition in schizophrenia. Psychiatry Res. 2007; 152:261–265.

35). Lin YT, Liu CM, Chiu MJ, Liu CC, Chien YL, Hwang TJ, et al. Differentiation of schizophrenia patients from healthy subjects by mismatch negativity and neuropsychological tests. PLoS One. 2012; 7:e34454.

36). Baldeweg T, Klugman A, Gruzelier J, Hirsch SR. Mismatch negativity potentials and cognitive impairment in schizophrenia. Schizophr Res. 2004; 69:203–217.

37). Light GA, Braff DL. Mismatch negativity deficits are associated with poor functioning in schizophrenia patients. Arch Gen Psychiatry. 2005; 62:127–136.

38). Kiang M, Light GA, Prugh J, Coulson S, Braff DL, Kutas M. Cognitive, neurophysiological, and functional correlates of proverb interpretation abnormalities in schizophrenia. J Int Neuropsychol Soc. 2007; 13:653–663.

39). Umbricht DS, Bates JA, Lieberman JA, Kane JM, Javitt DC. Electrophysiological indices of automatic and controlled auditory information processing in firstepisode, recent-onset and chronic schizophrenia. Biol Psychiatry. 2006; 59:762–772.

40). Light GA, Swerdlow NR, Braff DL. Preattentive sensory processing as indexed by the MMN and P3a brain responses is associated with cognitive and psychosocial functioning in healthy adults. J Cogn Neurosci. 2007; 19:1624–1632.

41). Naatanen R. Mismatch negativity: clinical research and possible applications. Int J Psychophysiol. 2003; 48:179–188.
42). Umbricht D, Javitt D, Novak G, Bates J, Pollack S, Lieberman J, et al. Effects of clozapine on auditory event-related potentials in schizophrenia. Biol Psychiatry. 1998; 44:716–725.

43). Umbricht D, Javitt D, Novak G, Bates J, Pollack S, Lieberman J, et al. Effects of risperidone on auditory event-related potentials in schizophrenia. Int J Neuropsychopharmacol. 1999; 2:299–304.

Fig. 1.
The grand averaged Mismatch negativity waveforms at Fz. A : Patients with schizophrenia. B : Healthy controls.
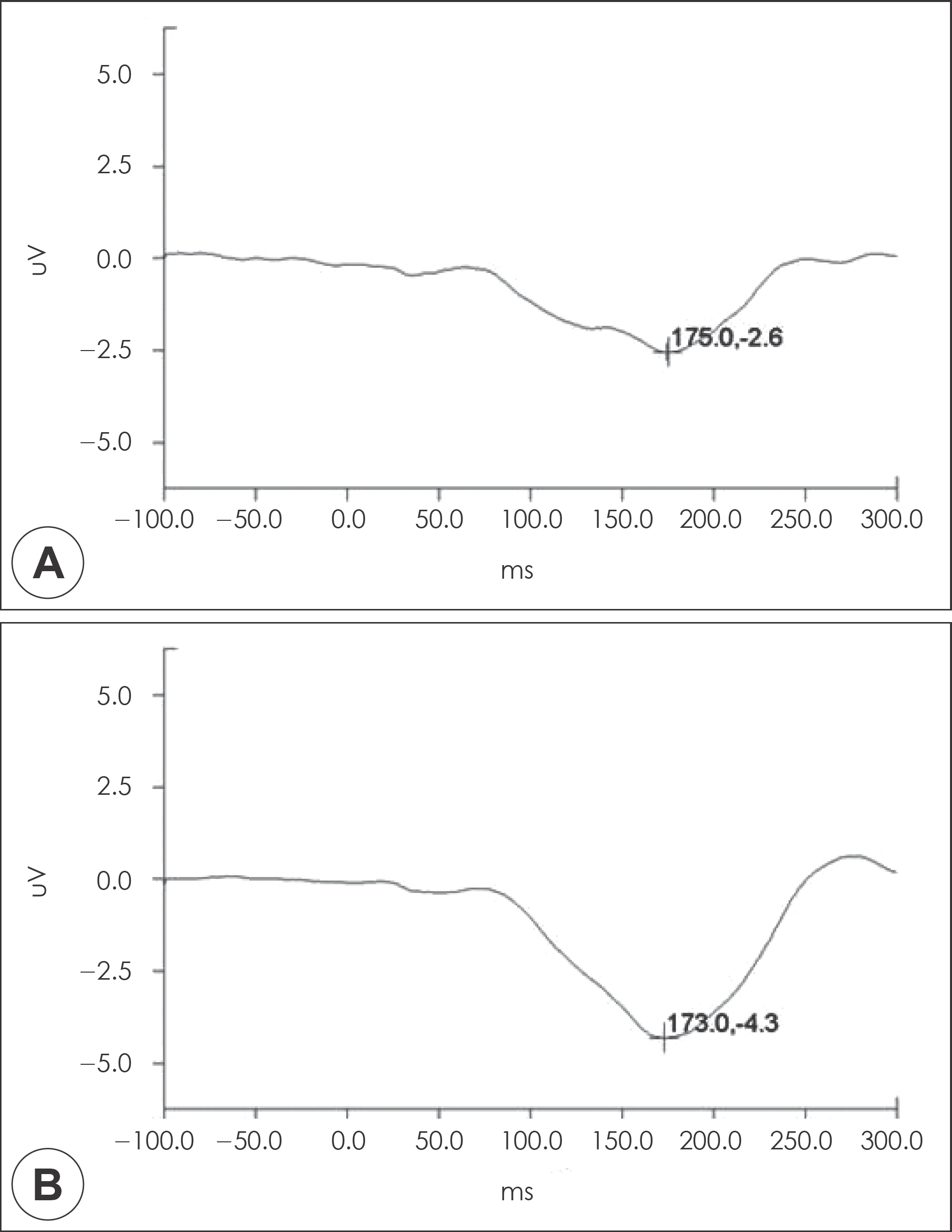
Fig. 2.
Mismatch negativity amplitude across group at Fz and Cz. Horizontal lines in the group indicate mean value and vertical lines in the group indicate 95% confidence interval.
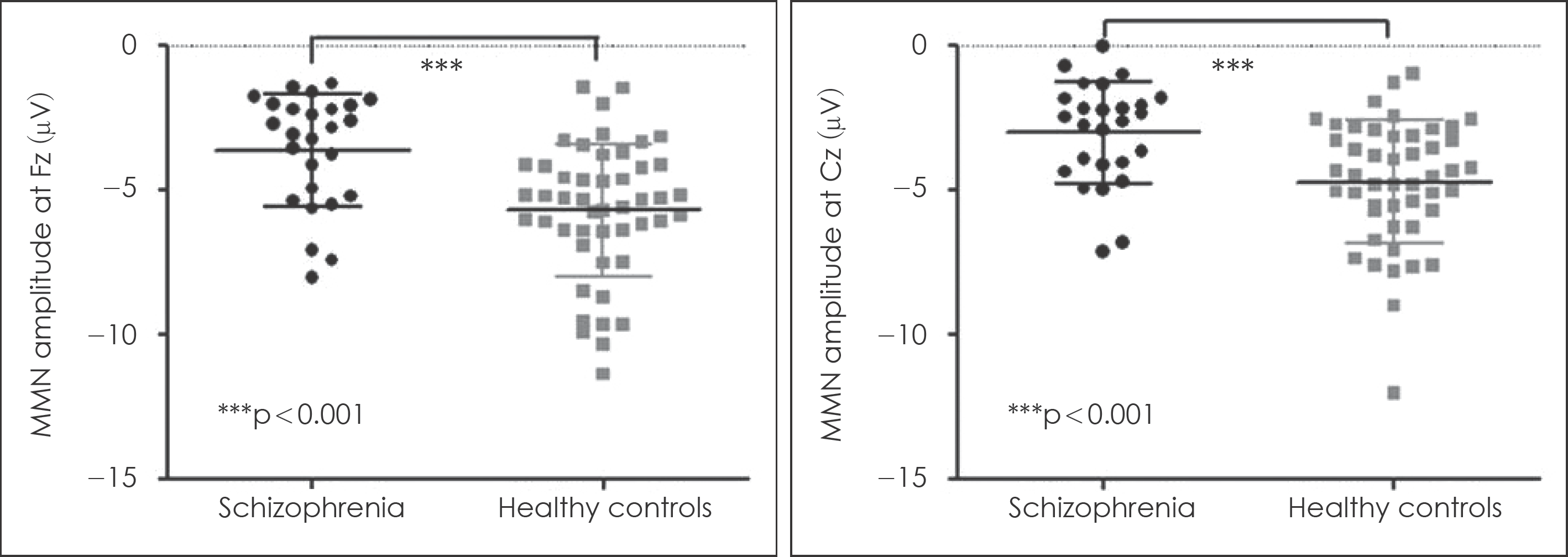
Fig. 3.
Correlation between Mismatch negativity amplitudes and subsets of short form of Korean Wechsler Adult Intelligence Scale in patients with schizophrenia.
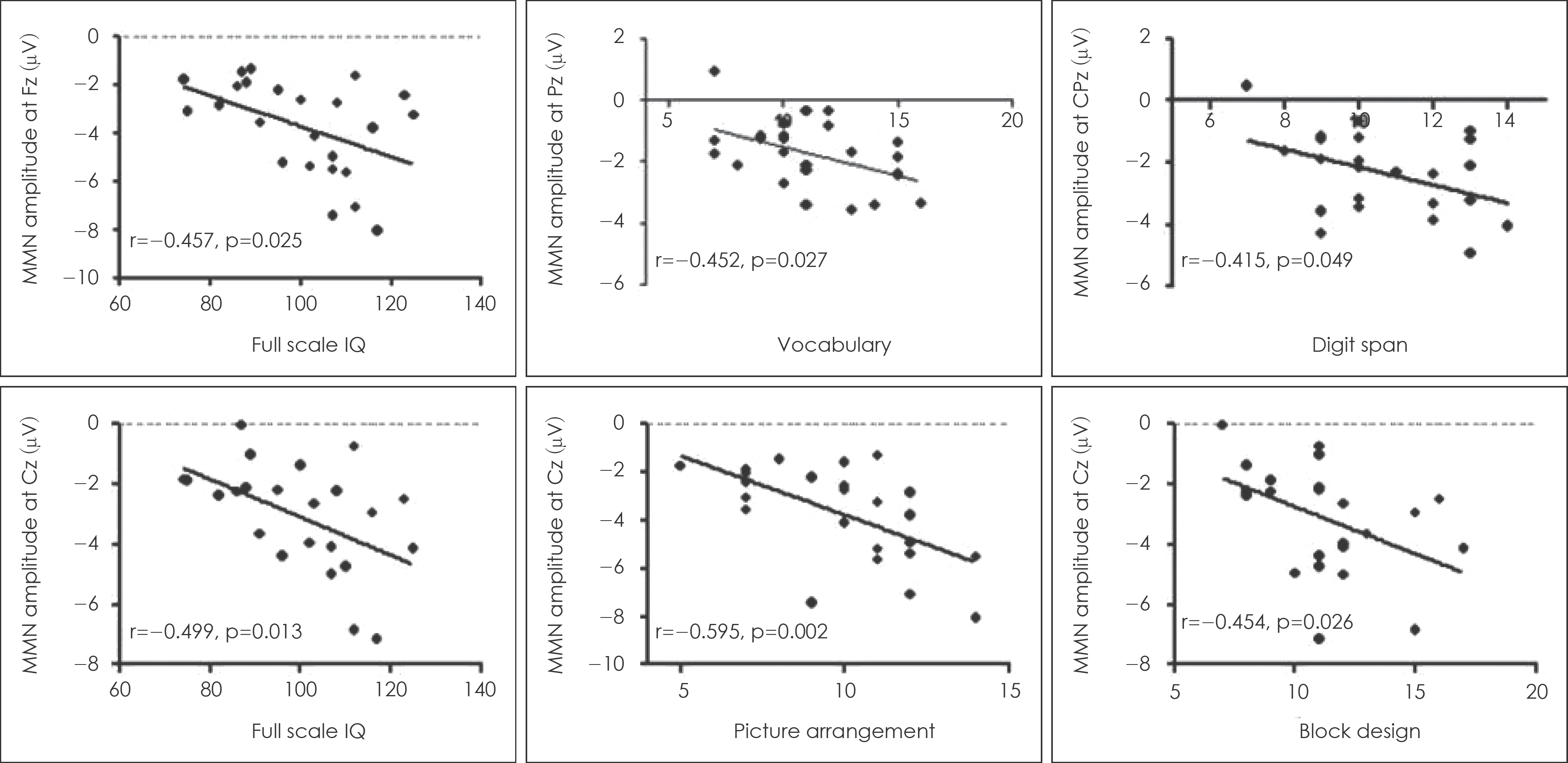
Fig. 4.
Correlation between Mismatch negativity amplitudes and Global Assessment of Functioning scores in patients with schizophrenia.
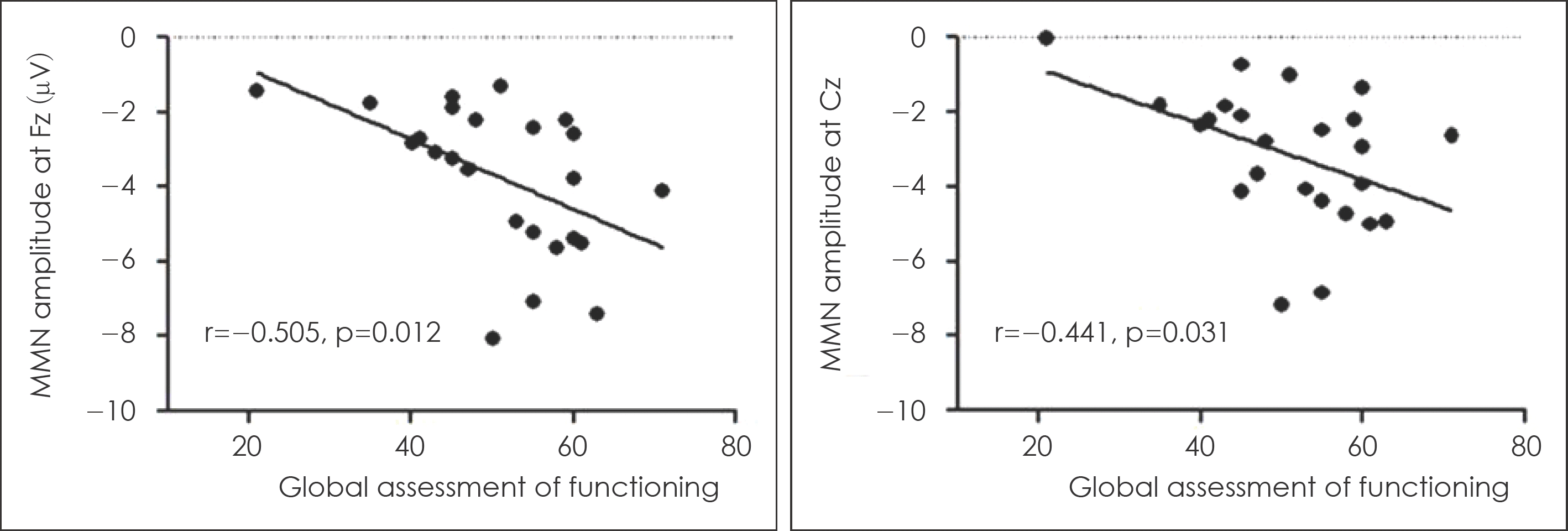
Table 1.
Demographic, neurocognitive and clinical characteristics of subjects
| Schizophrenia (N=26) | Healthy controls (N=48) | Analysis† | p value | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | χ2 or t | ||
| Demographic characteristics | ||||||
| Male/Female | 18/8 | 26/22 | 1.59 | 0.227 | ||
| Age (y) | 27.42 | 4.69 | 23.94 | 5.40 | 2.77 | 0.007∗ |
| Education (y) | 13.96 | 2.16 | 14.23∥ | 1.68 | -0.60 | 0.552 |
| Handedness (R/L) | 23/2‡ | 47/1 | 0.269 | |||
| Neurocognitive performances | ||||||
| WCST | ||||||
| Correct response | 65.21§ | 15.85 | 70.08 | 6.77 | -1.44 | 0.161 |
| Errors | 40.29§ | 30.00 | 16.15 | 7.57 | 3.88 | 0.001∗ |
| Perseverative response | 22.33§ | 19.74 | 8.54 | 4.27 | 3.38 | 0.002∗ |
| Non-perseverative response | 20.50§ | 16.84 | 8.08 | 4.44 | 3.55 | 0.002∗ |
| Perseverative error | 19.79§ | 16.03 | 8.10 | 3.78 | 3.52 | 0.002∗ |
| Categories achieved | 4.54§ | 2.15 | 5.92 | 0.58 | -3.08 | 0.005∗ |
| TMT-A | ||||||
| Time (sec)/error | 32.71/0.08§ | 12.15/0.28 | 21.81/0.15 | 6.22/0.36 | 4.13/-0.75 | <0.001∗/0.422 |
| TMT-B | ||||||
| Time (sec)/error | 93.29/0.46§ | 34.75/0.83 | 52.83/0.21 | 15.46/0.46 | 5.44/1.37 | <0.001∗/0.181 |
| COWA | ||||||
| Letters | 29.25§ | 8.89 | 44.44 | 10.09 | -6.26 | <0.001∗ |
| Categories | 29.00§ | 7.09 | 42.71 | 9.54 | -6.86 | <0.001∗ |
| K-WAIS (short form) | ||||||
| Vocabulary | 11.08§ | 2.65 | 12.54 | 2.01 | -2.37 | 0.023∗ |
| Digit span | 10.70‡ | 1.92 | 12.52 | 1.70 | -4.06 | <0.001∗ |
| Picture arrangement | 9.92§ | 2.39 | 11.63 | 1.84 | -3.35 | 0.001∗ |
| Block design | 11.21§ | 2.60 | 14.46 | 1.95 | -5.95 | <0.001∗ |
| Arithmatic | 10.17§ | 2.53 | 13.73 | 2.86 | -5.18 | <0.001∗ |
| Full scale iq | 100.50§ | 14.27 | 113.90 | 13.58 | -3.88 | <0.001∗ |
| Clinical characteristics | ||||||
| PANSS total | 57.08§ | 14.04 | ||||
| PANSS positive | 12.42§ | 4.12 | ||||
| PANSS negative | 16.25§ | 5.79 | ||||
| PANSS general | 28.42§ | 6.46 | ||||
| BPRS | 40.21§ | 8.31 | ||||
| GAF | 50.87§ | 10.69 | ||||
† : Independent t test or Welch's t test if the variances were not equal, χ2 analysis or Fisher's exact test for categorical data,
∥ : N=47, the number of missing data was 1. WCST : Wisconsin Card Sorting Test, TMT-A : Trail Making Test-A, TMT-B : Trail Making Test-B, COWA : Controlled Oral Word Association Test, K-WAIS : Korean Wechsler Adult Intelligence Scale, IQ : Intelligence Quotient, PANSS : Positive and Negative Syndrome Scale, BPRS : Brief Psychiatric Rating Scale, GAF : Global Assessment of Functioning, N : number of patients
Table 2.
Means and standard deviations of peak MMN amplitudes and latencies at central electrode sites
| Schizophrenia (N=26) | Healthy controls (N=48) | t | p value | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| Amplitude | ||||||
| FPZ | -3.75 | 2.00 | -4.69 | 2.10 | 1.86 | 0.067 |
| FZ | -3.61 | 1.95 | -5.70 | 2.29 | 3.92 | <0.001∗ |
| FCZ | -3.51 | 2.43 | -5.61 | 2.39 | 3.60 | <0.001∗ |
| CZ | -3.04 | 1.76 | -4.75 | 2.13 | 3.50 | 0.001∗ |
| CPZ | -2.30 | 1.28 | -3.71 | 1.76 | 3.57 | 0.001∗ |
| PZ | -1.68 | 1.08 | -2.61 | 1.27 | 3.20 | 0.002∗ |
| POZ | -1.28 | 1.12 | -1.57 | 1.03 | 1.11 | 0.271 |
| OZ | -0.90 | 0.91 | -1.14 | 1.54 | 0.73 | 0.469 |
| Latency | ||||||
| FPZ | 170.69 | 29.06 | 177.23 | 23.19 | -1.06 | 0.294 |
| FZ | 179.23 | 27.22 | 178.10 | 20.19 | 0.20 | 0.840 |
| FCZ | 174.42 | 30.90 | 177.10 | 18.58 | -0.41 | 0.688 |
| CZ | 175.92 | 31.17 | 176.06 | 19.09 | -0.02 | 0.984 |
| CPZ | 181.31 | 31.13 | 175.35 | 17.96 | 0.90 | 0.376 |
| PZ | 177.81 | 29.63 | 181.69 | 22.66 | -0.63 | 0.531 |
| POZ | 190.46 | 35.33 | 189.35 | 27.47 | 0.15 | 0.882 |
| OZ | 202.42 | 29.01 | 193.67 | 30.34 | 1.20 | 0.233 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download