Abstract
Objectives
Dental plaque emits red fluorescence under a visible blue light near the ultra-violet end of the light spectrum. The fluorescence characteristics of each microorganism have been reported in several studies. The aim of this study was to evaluate changes in red fluorescence of oral microorganisms that is affected by blood in the culture media.
Methods
The gram-positive Actinomyces naeslundii (AN, KCTC 5525) and Lactobacillus casei (LC, KCTC 3109) and gram negative Prevotella intermedia (PI, KCTC 3692) that are known to emit red fluorescence were used in this study. Each bacterium was activated in broth and cultivated in different agar media at 37℃ for 7 days. Tryptic soy agar with hemin and vitamin K3 (TSA), TSA with sheep blood (TSAB), basal medium mucin (BMM) medium, and BMM with sheep blood (BMMB) were used in this study. Fluorescence due to bacterial growth was observed under 405-nm wavelength blue light using the quantitative light-induced fluorescence-digital (QLF-D) device. The red, green, and blue fluorescence values of colonies were obtained using image-analysis software and the red to green ratio (R/G value) and red to total RGB ratio (R/RGB value) were calculated for quantitative comparison.
Results
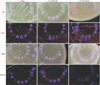
The QLF-D images of the AN, LC, and PI colonies showed red fluorescence in all media, but the fluorescence of all bacteria was reduced in TSA and BMM media, compared with in TSAB and BMMB media. Both the R/G and the R/RGB values of all bacteria were significantly reduced in growth media without blood (P<0.001).
Conclusions
Based on this in vitro study, it can be concluded that red fluorescence of oral bacteria can be affected by growth components, especially blood. Blood-containing medium could be a significant factor influencing red fluorescence of oral bacteria. It can be further hypothesized that bleeding in the oral cavity can increase the red fluorescence of dental plaque.
Figures and Tables
References
1. Kolenbrander PE, Palmer RJ, Periasamy S, Jakubovics NS. Oral multispecies biofilm development and the key role of cell - cell distance. Nat Rev Microbiol. 2010; 8:471–480.

2. Zijnge V, van Leeuwen MB, Degener JE, Abbas F, Thurnheer T, Gmür R, Harmsen HJ. Oral biofilm architecture on natural teeth. PloS one. 2010; 5(2):e9321.

3. van der Veen MH, de Josselin de Jong E. Application of quantitative light-induced fluorescence for assessing early caries lesions. Monogr Oral Sci. 2000; 17:144–162.

4. Coulthwaite L, Pretty IA, Smith PW, Higham SM, Verran J. The microbiological origin of fluorescence observed in plaque on dentures during QLF analysis. Caries Res. 2006; 40:112–116.

5. Koenig K, Schneckenburger H, Hemmer J, Tromberg BJ, Steiner RW. In-vivo fluorescence detection and imaging of porphyrin-producing bacteria in the human skin and in the oral cavity for diagnosis of acne vulgaris, caries, and squamous cell carcinoma. Int Soc Opt Photon. 1994; 2135:129–139.
6. Fyrestam J, Bjurshammar N, Paulsson E, Johannsen A, Östman C. Determination of porphyrins in oral bacteria by liquid chromatography electrospray ionization tandem mass spectrometry. Anal Bioanal Chem. 2015; 407:7013–7023.

7. Thomas RZ, van der Mei HC, van der Veen MH, de Soet JJ, Huysmans MCDNJM. Bacterial composition and red fluorescence of plaque in relation to primary and secondary caries next to composite: an in situ study. Oral Microbiol Immunol. 2008; 23:7–13.

8. van der Veen MH, Thomas RZ, Huysmans MCDNJM, de Soet JJ. Red autofluorescence of dental plaque bacteria. Caries Res. 2006; 40:542–545.

9. Kim YS, Lee ES, Kwon HK, Kim BI. Monitoring the maturation process of a dental microcosm biofilm using the Quantitative Light-induced Fluorescence-Digital (QLF-D). J Dent. 2014; 42:691–696.

10. Lee ES, Kang SM, Ko HY, Kwon HK, Kim BI. Association between the cariogenicity of a dental microcosm biofilm and its red fluorescence detected by Quantitative Light-induced Fluorescence-Digital (QLF-D). J Dent. 2013; 41:1264–1270.

11. Lennon AM, Buchalla W, Brune L, Zimmermann O, Gross U, Attin T. The ability of selected oral microorganisms to emit red fluorescence. Caries Res. 2006; 40:2–5.

12. Volgenant CMC, van der Veen MH, de Soet JJ, ten Cate JM. Effect of metalloporphyrins on red autofluorescence from oral bacteria. Eur J Oral Sci. 2013; 121:156–161.

13. Zhang L. Heme Biology: The Secret Life of Heme in Regulating Divers Biological Processes. 1st ed. Singapore: World Scientific;2011. p. 1–6.
14. Wong L, Sissions CH. A comparison of human dental plaque microcosm biofilms grown in an undefined medium and a chemically defined artificial saliva. Arch Oral Biol. 2001; 46:477–486.

15. Kim YS, Kang SM, Lee ES, Lee JH, Kim BR, Kim BI. Ecological changes in oral microcosm biofilm during maturation. J Biomed Opt. 2016; 21:101409–101409.

16. Naver corp. Terms.naver.com [Internet]. cited 2017 Nov 013. Available from: http://terms.naver.com/entry.nhn?docId=3340383&cid=58161&categoryId=58161.
17. Kim BI. QLF Concept and Clinical Implemezntation. J Korean Dent Assoc. 2011; 49:443–450.
18. Hwang HR, Cho YS, Kim BI. Assessment of Clinical Applicability of a New Plaque Scoring System Using Quantitative Light-Induced Fluorescence-Digital. J Dent Hyg Sci. 2014; 14:150–157.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download