Abstract
Objectives
The purpose of the present study was to evaluate the whitening effect, morphological and structural changes, and remineralization of the enamel induced by 3 combined agents: amorphous calcium phosphate (ACP), hydroxyapatite (HA), and tetrasodium pyrophosphate (TSP).
Methods
The study was performed on 90 bovine enamel slabs, which were divided into the 6 groups: negative control-distilled water (Group 1); positive control-opalescence F (Group 2); 10% mixed agent (Group 3); 25% mixed agent (Group 4); 50% mixed agent (Group 5); and 100% mixed agent (Group 6). Changes in the shade of the enamel slabs were evaluated using Shade Eye-NCC. Morphological changes were assessed by scanning electron microscopy (SEM) and confocal laser scanning microscopy (CLSM) was used to determine the remineralizing effect of the three agents on enamel slabs.
Results
The change in shade of the enamel (ad*) was noted to increase significantly with increase in whitening frequency in all groups. The value of Δn* was significantly greater in all groups except for the negative control group (P<0.001). SEM revealed that the control group, Group 5, and Group 6 had similar morphologies. The fluorescence lesion areas in the 4 mixture-treated group were significantly smaller than those in the positive control group (P<0.001).
Conclusions
These results showed that the mixture of ACP, HA, and TSP was highly effective for bovine enamel whitening and acted by inducing the remineralization of enamel. Clinical significance: We evaluated the applicability of a new mixture containing ACP, HA, and TSP. This mixture would be highly useful in aesthetic dentistry because of its whitening efficiency, which does not compromise the enamel's integrity.
Go to : 
References
1. Badole GP, Warhadpande MM, Bahadure RN, Badole SG. Aesthetic rehabilitation of discoloured nonvital anterior tooth with carbamide peroxide bleaching: case series. J Clin Diagn Res. 2013; 7:3073–3076.

3. Sulieman MA. An overview of tooth-bleaching techniques: chemistry, safety and efficacy. Periodontol. 2008; 48:148–169.
4. Hilgenberg SP, Pinto SC, Farago PV, Santos FA, Wambier DS. Physical-chemical characteristics of whitening toothpaste and evaluation of its effects on enamel roughness. Braz Oral Res. 2011; 25:288–294.

5. Singh RD, Ram SM, Shetty O, Chand P, Yadav R. Efficacy of casein Phosphopeptidee-amorphous calcium phosphate to prevent stain absorption on freshly bleached enamel: An in vitro study. J Conserv Dent. 2010; 13:76–79.
6. Featherstone JD. Remineralization, the natural caries repair process–the need for new approaches. Adv Dent Res. 2009; 21:4–7.

7. Schlesinger DH, Hay DI. Complete covalent structure of statherin, a tyrosine-rich acidic peptide which inhibits calcium phosphate precipitation from human parotid saliva. J Biol Chem. 1977; 252:1689–1695.

8. Cunha AG, De Vasconcelos AA, Borges BC, Vitoriano Jde O, Alves-Junior C, Machado CT, et al. Efficacy of in-office bleaching techniques combined with the application of a casein phosphopeptide-amorphous calcium phosphate paste at different moments and its influence on enamel surface properties. Microsc Res Tech. 2012; 75:1019–1025.
9. DE Abreu DR, Sasaki RT, Amaral FL, Flório FM, Basting RT. Effect of home-use and in-office bleaching agents containing hydrogen peroxide associated with amorphous calcium phosphate on enamel microhardness and surface roughness. J Esthet Restor Dent. 2011; 23:158–168.

10. Goo Hyo-Jin, Kwun Hyeon-Sook, Park Jeong-Hee, Cho Min-Jeong, Kim Eun-Kyong, Choi Youn-Hee, et al. Effect of fluoride application after tooth bleaching using the diode Laser. J Korean Academy Oral Health. 2008; 32:160–169.
11. Borges BC, Pinheiro MH, Feitosa DA, Correia TC, Braz R, Montes MA, et al. Preliminary study of a novel in-office bleaching therapy modified with a casein phosphopeptide-amorphous calcium phosphate. Microsc Res Tech. 2012; 75:1571–1575.

12. Borges BC, Pinheiro MH, Feitosa DA, Correia TC, Braz R, Montes MA, et al. Effect of a nano-hydroxyapatite paste on bleaching-related tooth sensitivity. J Esthet Restor Dent. 2012; 24:268–276.
13. Dabanoglu A, Wood C, García-Godoy F, Kunzelmann KH. Whitening effect and morphological evaluation of hydroxyapatite materials. Am J Dent. 2009; 22:23–29.
14. Besinis A, van Noort R, Martin N. Infiltration of demineralized dentin with silica and hydroxyapatite nanoparticles. Dent Mater. 2012; 28:1012–1023.

15. Swarup JS, Rao A. Enamel surface remineralization: Using synthetic nanohydroxyapatite. Contemp Clin Dent. 2012; 3:433–436.

16. Huang S, Gao S, Cheng L, Yu H. Remineralization potential of nano-hydroxyapatite on initial enamel lesions: an in vitro study. Caries Res. 2011; 45:460–468.

17. Lobene RR. A clinical study of the anticalculus effect of a dentifrice containing soluble pyrophosphate and sodium fluoride. Clin Prev Dent. 1986; 8:5–7.
18. Schemehorn BR, Moore MH, Putt MS. Abrasion, polishing, and stain removal characteristics of various commercial dentifrices in vitro. J Clin Dent. 2011; 22:11–18.
19. Farrell S, Barker ML, Gerlach RW, Putt MS, Milleman JL. Prevention of lingual calculus formation with daily use of 6% H2O2/2% pyrophosphate whitening strips. J Clin Dent. 2009; 20:75–78.
20. Khantee S, Patanapiradej V, Maneenut C, Tantbirojn D. Effect of acidic food and drinks on surface hardness of enamel, dentine, and tooth-coloured filling materials. J Dent. 2006; 34:214–220.
21. Lee YE, Baek HJ, Choi YH, Jeong SH, Park YD, Song KB. Comparison of remineralization effect of three topical fluoride regimens on enamel initial carious lesions. J Dent. 2010; 38:166–171.

22. Paris S, Meyer-Lueckel H, Mueller J, Hummel M, Kielbassa AM. Progression of sealed initial bovine enamel lesions under demineral-izing conditions in vitro. Caries Res. 2006; 40:124–129.

24. Eimar H, Siciliano R, Abdallah MN, Nader SA, Amin WM, Martinez PP, et al. Hydrogen peroxide whitens teeth by oxidizing the organic structure. J Dent. 2012; 40:e25–33.

25. Mor C, Steinberg D, Dogan H, Rotstein I. Bacterial adherence to bleached surfaces of composite resin in vitro. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998; 86:582–586.

26. Morrier JJ, Duprez JP, Boulet O. Enamel, composites and Coca-cola. Rev Odontostomatol. 1989; 18:93–98.
27. Bayindir F, Kürklü D, Yanikoğlu ND. The effect of staining solutions on the color stability of provisional prosthodontic materials. J Dent. 2012; 40:e41–e46.

28. Ergün G, Mutlu-Sagesen L, Ozkan Y, Demirel E. In vitro color stability of provisional crown and bridge restoration materials. Dent Mater J. 2005; 24:342–350.
Go to : 
 | Fig. 1.Morphology of the superficial enamel surface after 6 days of treatment in each group (A: Group 1, B: Group 2, C: Group 3, D: Group 4, E: Group 5, F: Group 6) (×2,000). |
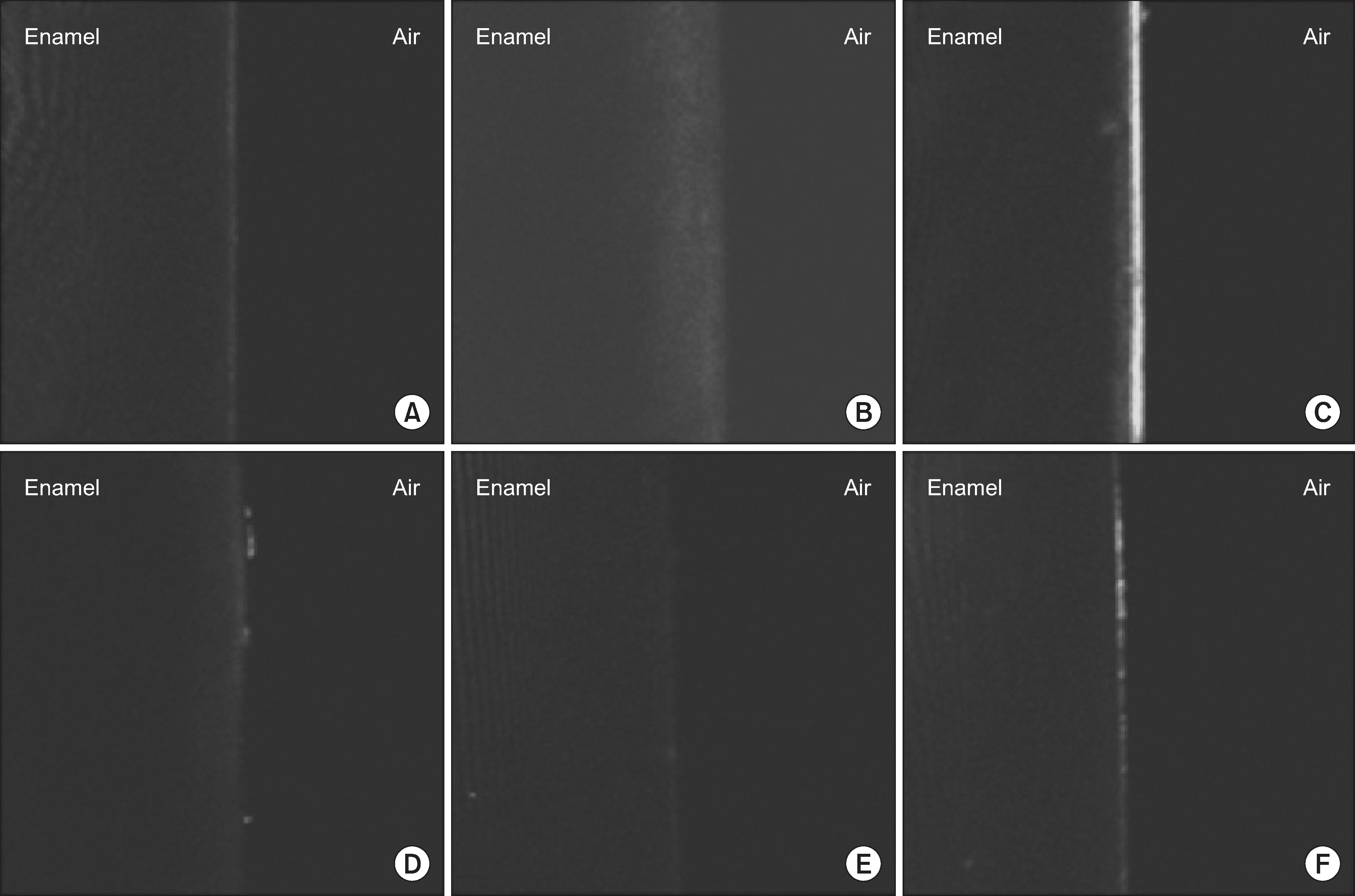 | Fig. 2.Fluorescent lesions after whitening in each group at 6 days as determined by CLSM; note the demineralized lesion in the center, emits a bright color (A: Group 1, B: Group 2, C: Group 3, D: Group 4, E: Group 5, F: Group 6). |
Table 1.
Study group and characteristics of the treatment materials
Table 2.
Shade change (ΔE*) of the specimen according to the whitening times
| Group | After 1 day* | After 2 days* | After 3 days* | After 4 days* | After 5 days* | After 6 days* |
|---|---|---|---|---|---|---|
| Group 1† | 6.93±2.49a | 6.13±2.41a | 8.38±3.28a | 7.77±1.70a | 8.37±1.53a | 9.72±1.35a |
| Group 2† | 30.4±7.55b | 39.92±8.53b | 47.45±8.14b | 51.21±7.12b | 51.07±6.24b | 54.72±7.14b |
| Group 3† | 21.2±4.11c | 26.37±4.55c | 31.52±4.38c | 34.37±3.71c | 34.42±1.86c | 37.30±1.73c |
| Group 4† | 25.24±3.61bc | 33.09±5.39bc | 35.75±5.81c | 36.18±6.17c | 38.58±5.54c | 31.94±1.92c |
| Group 5† | 26.65±2.89b | 34.22±3.80b | 38.78±2.87c | 41.09±2.94c | 41.32±3.04c | 42.92±0.92c |
| Group 6† | 28.90±3.63b | 34.75±4.86b | 37.46±3.81c | 41.21±2.54c | 42.00±3.87bc | 45.72±4.02c |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download