Abstract
Objectives
The purpose of this study was to evaluate the growth inhibitory effects of some vegetable oils on Streptococcus mutans (S. mutans) and Lactobacillus casei (L. casei).
Methods
Two bacterial strains and 5 kinds of test solutions (3 experimental groups: orange essential oil, olive oil, soybean oil; 1 positive control group: chlorhexidine solution; 1 negative control group: broth medium) were used in this study. S. mutans and L. casei pellets were exposed to 1 ml of one of the test solutions for 1 minute. Then, the treated bacterial cells were incubated in fresh broth medium for 0, 4, 8, 16, and 24 hours. The optical density of the broth medium was measured using an ELISA reader at 620 nm. A nonparametric Kruskal-Wallis test (with Mann-Whitney U tests) was performed to compare the change in optical density between different groups at different time points.
Results
Bacterial growth was significantly inhibited in all experimental groups compared to the negative control group. The growth of L. casei was less affected by experimental oils than that of S. mutans. Orange essential oil had the maximum growth inhibitory effect on S. mutans up to 8 hours, similar to that in the positive control group (P<0.01). Experimental oils had greater growth inhibitory effect on L. casei than chlorhexidine solution.
Go to : 
References
1. Paster BJ, Olsen I, Aas JA, Dewhirst FE. The breadth of bacterial diversity in the human periodontal pocket and other oral sites. Peri-odontol 2000. 2006; 42:80–87.

3. Song YJ. Classification of Periodontal Pathogens Based on Genetic Specificity[master’s thesis]. Seoul: Seoul National University;2014. [Korean].
5. Kim EJ. Study on the reduction effects on oral microorganisms through the different methods of controlling dental plaque[master’s thesis]. Seoul: Dankook University;2003. [Korean].
6. Filoche SK, Soma K, Sissons CH. Antimicrobial effects of essential oils in combination with chlorhexidine digluconate. Oral Microbiol Immunol. 2005; 20:221–225.

7. Palombo EA. Traditional medicinal plant extracts and natural products with activity against oral bacteria: potential application in the prevention and treatment of oral diseases. Evid Based Complement Alternat Med. 2011; 2011:1–15.

8. Trahan L. Xylitol: a review of its action on mutans streptococci and dental plaque-its clinical significance. Int Dent J. 1995; 45:77–92.
9. Hwang JK, Shim JS, Baek NI, Pyun YR. Xanthorrhizol: a potential antibacterial agent from Curcuma xanthorrhiza against Streptococcus mutans. Planta Med. 2000; 66:196–197.
10. Baratta MT, Dorman HJ, Deans SG, Figueiredo AC, Barroso JG, Ruberto G. Antimicrobial and antioxidant properties of some commercial essential oils. Flavour Fragr J. 1998; 13:235–244.

11. Lee SY, Kim JK, Baek BJ, Yang YM, Lee KY, Lee YH, et al. Antibacterial effect of essential oils on oral bacteria. J Korean Acad Pediatr Dent. 2009; 36:1–11.
12. Takarada K, Kimizuka R, Takahashi N, Honma K, Okuda K, Kato T. A comparison of the antibacterial efficacies of essential oils against oral pathogens. Oral Microbiol Immunol. 2004; 19:61–64.

13. Lim DJ. Extraction of natural citrus by SC-CO2 and their pharmacological effect on aroma therapy[docter of philosophy]. Busan: Pukyong National University;2014. [Korean].
14. Fife B. Oil pulling therapy : detoxifying and healing the body through oral cleansing. Seoul: Piccadilly Books;2013. p. 274–280.
15. Tomar P, Hongal S, Jain M, Rana K, Saxena V. Oil Pulling and Oral Health: A Review. IJSS. 2014; 1:33–37.
16. Asokan S, Rathan J, Muthu MS, Rathna PV, Emmadi P, Raghura-man , et al. Effect of oil pulling on Streptococcus mutans count in plaque and saliva using Dentocult SM Strip mutans test: a randomized, controlled, triple-blind study. J Indian Soc Pedod Prev Dent. 2008; 26:12–17.
17. Amith HV, Ankola AV, Nagesh L. Effect of oil pulling on plaque and gingivitis. J Oral Health Comm Dent. 2007; 1:12–18.
18. Kim JH. Antiocidant and antimicrobial effects of lemon and eucalyptus essential oils[master’s thesis]. Seoul: Konkuk University;2011. [Korean].
19. Kang SM. Effect of essential oil mouthrinse on oral squamous epithelial cells (KB Cells)[master’s thesis]. Seoul: Chung-ang Univer- sity;2010. [Korean].
20. Kim SH, Lim SA, Park SJ, Kim DK. Assessment oral Health-related quality of life using the Oral Health Impact Profile(OHIP). J Korean Acad Oral Health. 2004; 28:559–569.
21. Bakkali F, Averbeck S, Averbeck D, Idaomar M. Biological effects of essential oils-a review. Food Chem Toxicol. 2008; 46:446–475.
22. Shapiro S, Meier A, Guggenheim B. The antimicrobial activity of essential oils and essential oil components towards oral bacteria. Oral Microbiol Immunol. 1994; 9:202–208.

23. Ouhayoun JP. Penetrating the plaque biofilm: impact of essential oil mouthwash. J Clin Periodontol. 2003; 30:10–12.

24. Charles CH, Mostler KM, Bartels LL, Mankodi SM. Comparative an-tiplaque and antigingivitis effectiveness of a chlorhexidine and an essential oil mouthrinse: 6-month clinical trial. J Clin Periodontol. 2004; Oct. 31(10):878–84.

25. Scheie AA, Rukke HV, Perterson FC. Are antibacterials necessary in caries prophylaxis? Fejerskov O, Nyvad B, Kidd E, editors. Dental caries: the disease and its clinical management. 3rd ed.Oxford: Wiley Blackwell;2015. p. 287–301.
26. Hugo WB, Longworth AR. Some aspects of the mode of action of chlorhexidine. J Pharm Phamacol. 1964; 16:655–662.

27. Emilson CG. Susceptibility of various microorganisms to chlorhexidine. Scand J Dent Res. 1977; 85:255–265.

29. Joyston-Bechal S, Hayes K. Davenport ES, Hardie JM. Caries incidence, mutans streptococci and lactobacilli in irradiated patients during a 12-month preventive programme using chlorhexidine and fluoride. Caries Res. 1992; 26:384–390.
Go to : 
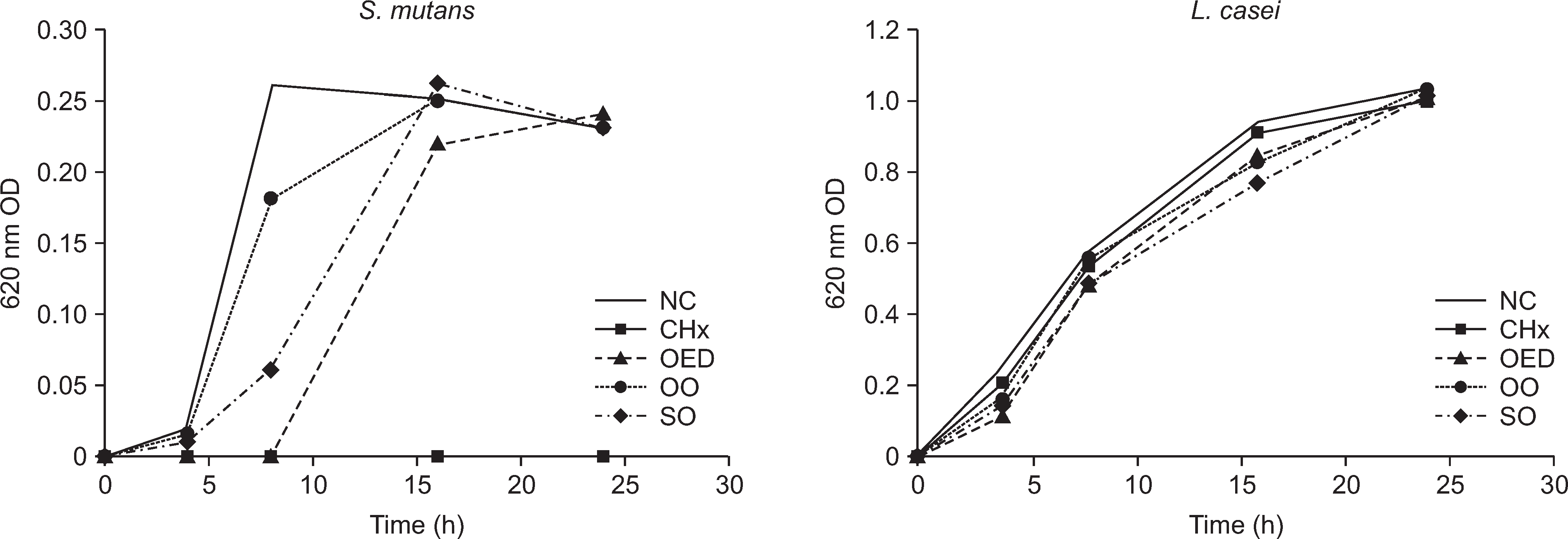 | Fig. 2.The result of growth curve on S. mutans and L. casie for 0, 4, 8, 16, 24 hours. NC: Negative control, CHx: hexamedine solution, LS: listerine zero, OO: olive oil, OEO: essential oil, SO: soybean oil. |
Table 1.
Test solution of this study
Table 2.
Optical density (620 nm) of S. mutans according to the test solution per time (Mean±SD)
| N | 0 h | 4 h | 8 h | 16 h | 24 h | P-value† | |
|---|---|---|---|---|---|---|---|
| NC | 9 | 0.00±0.00a | 0.02±0.01bA | 0.26±0.02cA | 0.25±0.01cA | 0.23±0.01dA | P<0.001 |
| CHx | 9 | 0.00±0.00a | 0.00±0.00aB | 0.00±0.00aB | 0.00±0.00aB | 0.00±0.01aB | P=0.048 |
| OEO | 9 | 0.00±0.00a | 0.00±0.01aBC | 0.00±0.01aB | 0.22±0.01bC | 0.24±0.01bA | P<0.001 |
| OO | 9 | 0.00±0.00a | 0.01±0.00bAD | 0.18±0.01cC | 0.25±0.01dA | 0.23±0.01eA | P<0.001 |
| SO | 9 | 0.00±0.00a | 0.01±0.01aBD | 0.06±0.01bD | 0.26±0.01cA | 0.23±0.01dA | P<0.001 |
| P-value‡ | P=1.000 | P<0.001 | P<0.001 | P<0.001 | P<0.001 | ||
Table 3.
Optical density (620 nm) of L. casei according to the test solution per time (Mean±SD)
| N | 0 h | 4 h | 8 h | 16 h | 24 h | P-value† | |
|---|---|---|---|---|---|---|---|
| NC | 9 | 0.00±0.00a | 0.26±0.01bA | 0.59±0.06cA | 0.94±0.06dA | 1.03±0.03eAB | P<0.001 |
| CHx | 9 | 0.00±0.00a | 0.21±0.01bB | 0.54±0.08cAC | 0.91±0.08dAB | 1.00±0.01dA | P<0.001 |
| OEO | 9 | 0.00±0.00a | 0.12±0.02bC | 0.49±0.09cBC | 0.85±0.09dACD | 1.01±0.02eAB | P<0.001 |
| OO | 9 | 0.00±0.00a | 0.17±0.03bD | 0.56±0.06cAB | 0.83±0.06dBC | 1.03±0.02eB | P<0.001 |
| SO | 9 | 0.00±0.00a | 0.15±0.01bCD | 0.49±0.04cB | 0.77±0.04dCD | 1.01±0.01eAB | P<0.001 |
| P-value‡ | P=1.000 | P<0.001 | P<0.001 | P<0.001 | P<0.05 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download