Abstract
Objectives
This study was conducted to investigate the influence of several commercial red ginseng beverages on the surface of healthy teeth and to confirm the anti-erosive effect of added calcium.
Methods
For the experimental group selection, the pH of red ginseng beverages on the market were measured and the mean pH was calculated. Beverages with the lowest pH (Dong Wha Hongsam Gold; red ginseng beverage group with pH 2.98), mid-level pH (Kwangdong Jin Hongsam Gold; red ginseng beverage group with pH 3.61), and the highest pH (Hongsam Han Ppuri; red ginseng beverage group with pH 5.34) were selected as the experimental groups. In order to confirm the anti-erosive effect of added calcium, we added 1% calcium to the product with the lowest pH (red ginseng beverage group with pH 2.98+1% Ca) and included the product in the experimental group. Jeju Samdasoo and Coca Cola were used as the negative and positive control groups, respectively. We soaked healthy bovine teeth samples in the selected six beverages for 1, 3, 5, 10, 15, and 30 minutes. The surface microhardness (VHN, Vickers hardness number) and the surface roughness (center line average roughness, Ra) of each sample were measured, and the surface features were observed with a scanning electron microscope (SEM).
Results
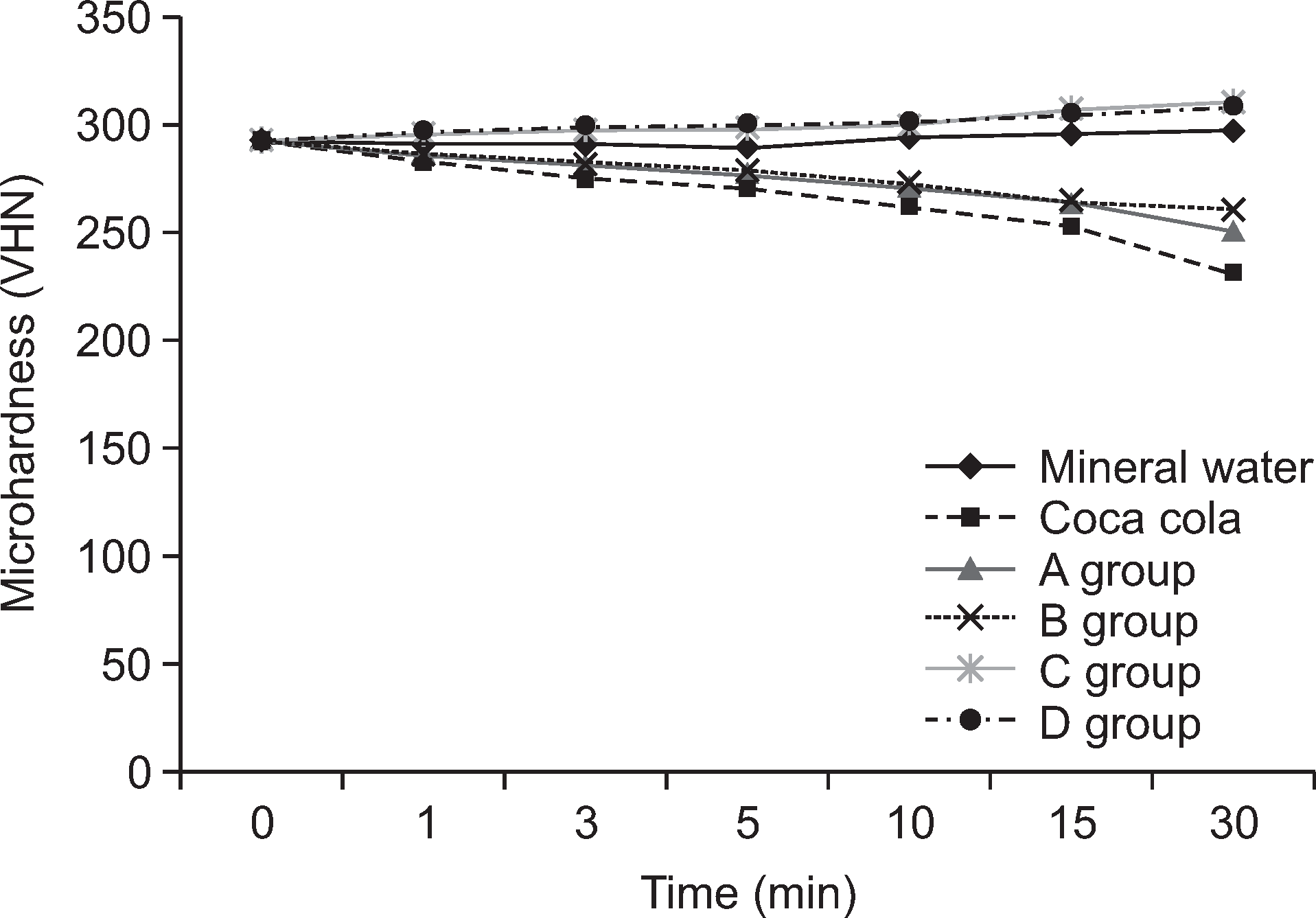
The change in the surface micro-hardness (△VHN) of teeth after 30 minutes of soaking in each beverage was the highest in the positive control group (60.99±8.99), followed by A (41.63±8.96), B (30.64±8.21), and the negative control group (―4.48±7.29) (P<0.05). No significant difference was observed in group C (―18.79±10.11) or D (―16.40±7.89). Surface roughness (Ra) exhibited significant differences between each group (P<0.05). Surface roughness (Ra) was high in A (102.88±26.34) and B (67.76±39.89), as well as in the positive control group (101.21±39.59). In contrast, C (30.80± 28.49) and D (25.05±10.79) showed low surface roughness values similar to the negative control group (23.77±22.48). Following SEM examination, severe cracks were observed between the crystals in groups A and B; such characteristics were similar to those of the positive control group.
Conclusions
Red ginseng beverages with low pH were shown to erode the surface of the teeth. When calcium was added to the red ginseng beverages, a decrease in tooth erosion was observed. Therefore, the possibility of tooth erosion should be considered when drinking red ginseng beverages. Furthermore, the addition of calcium to red ginseng beverages can be an alternative solution to suppress tooth erosion.
Go to : 
References
1. Ministry of Food and Drug Safety. 2015 Food&Drug Statistical Yearbook. cheongju: Ministry of Food and Drug Safety;2015. p. 191.
2. Cui Y, Shu XO, Gao YT, Cai H, Tao MH, Zheng W. Association of ginseng use with survival and quality of life among breast cancer patients. Am J Epidemiol. 2006; 163:645–653.

3. Block KI, Mead MN. Immune system effects of echinacea, ginseng, and astragalus: a review. Integr Cancer Ther. 2003; 2:247–267.

4. Huong NT, Murakami Y, Tohda M, Watanabe H, Matsumoto K. Social isolation stress-induced oxidative damage in mouse brain and its modulation by majonoside-R2, a Vietnamese ginseng saponin. Biol Pharm Bull. 2005; 28:1389–1393.

5. Jae MH, Chang KW, Ma DS. The effects of origanum oil, red ginseng extract, and green tea extract on oral microorganisms and volatile sulfur compounds. J Korean Acad Oral Health. 2011; 35:396–404.
6. Dictionary of Food Science and Technology. Korean Socity of Food Science and Technology. 2008. 1067.
7. Ministry of Agriculture, Food and Rural Affairs[Internet]. Newswire. [cited 2015 Nov 25]. available from:http://www.mafra.go.kr/main.jsp.
8. Choi DY, Shin SC. A study on pH of several beverages in Korea. J Korean Acad Oral Health. 1996; 20:399–410.
9. Imfeld T. Dental erosion: Definition, classification and links. Eur J Oral Sci. 1996; 104:151–155.

10. Scheutzel P. Etiology of dental erosion intrinsic factors. Eur J Oral Sci. 1996; 104:178–190.
11. Zero DT. Etiology of dental erosion-extrinsic factors. Eur J Oral Sci. 1996; 104:162–177.
13. Attin T, Weiss K, Becker K, Buchalla W, Wiegand A. Impact of modified acidic soft drinks on enamel erosion. Oral Dis. 2005; 11:7–12.

14. Sánchez GA, Fernandez De Preliasco MV. Salivary pH changes during soft drinks consumption in children. Int J Paediatr Dent. 2003; 13:251–257.

15. Larsen MJ, Nyvad B. Enamel erosion by some soft drinks and orange juices relative to their pH, buffering effect and contents of calcium phosphate. Caries Res. 1999; 33:81–87.

16. Jensdottir T, Bardow A, Holbrook P. Properties and modification of soft drinks in relation to their erosive potential in vitro. J Dent. 2005; 33:569–575.

17. Linnett V, Seow WK. Dental erosion in children : a literature review. pediatr Dent. 2001; 23:37–43.
18. Ehlen LA, Marshall TA, Qian F, Wefel JS, Warren JJ. Acidic beverages increase the risk of in vitro tooth erosion. Nutr Res. 2008; 28:299–303.

19. Youn HJ. Surface microhardness changes caused by commercial drinks on sound enamel of bovine teeth [Mater’s thesis]. Gwangju: Chonnam National University;2006. [Korean].
20. West NX, Hughes JA, Addy M. The effect of pH on the erosion of dentin and enamel by dietary acids in vitro. J Oral Rehabil. 2001; 28:860–864.
21. Hall AF, Buchanan CA, Millett DT, Creanor SL, Strang R, Foye RH. The effect of saliva on enamel and dentine erosion. J Dent. 1999; 27:333–339.

22. Attin T, Meyer K, Hellwing E, Buchallar W, Lennon AM. Effect of mineral supplements to citric acid on enamel erosion. Arch Oral Bio. 2003; 48:753–759.

23. Lee HJ. Erosive effect of hangover beverages on bovine teeth[Mater’ s thesis]. Gwangju: Chonnam National University;2011. [Korean].
24. Hicks J, Garcia-Godoy F, Flaitz C. Biological factors in dental caries: role of saliva and dental plaque in the dynamic process of demineralization and remineralization. J Clin Pediatr Dent. 2004; 28:47–52.
25. Kim KH. The effects of fermented milks on sound enamel surface[Master’s thesis]. Gwangju: Chonnam National Univer-sity;2014. [Korean].
26. Hong HS. Effects of red vinegar drink on oral pathogenic microbes and enamel surface [Master’s thesis]. Gwangju: Chonnam National University;2015. [Korean].
Go to : 
 | Fig. 2.SEM image of enamel surface after treatment on enamel (1: Mineral water, 2: Coca cola, 3: A group 4: B group, 5: C group, 6: D group, A: × 10,000, B: ×50,000). |
Table 1.
Drinks used in the experiment
Table 2.
The pH of experiment groups in this study
| Group | pH |
|---|---|
| Mineral water | 7.35±0.02 |
| Coca cola | 2.43±0.09 |
| A | 2.98±0.01 |
| B | 3.61±0.01 |
| C | 5.34±0.01 |
| D | 4.11±0.03 |
Table 3.
Differences of surface microhardness after treatment for 30 minutes Unit: VHN
| Group | N |
Time |
Difference† | |
|---|---|---|---|---|
| Before treatment | After treatment | |||
| Mineral water | 12 | 292.73±8.44 | 297.21±6.27 | 4.48±7.29b |
| Coca cola* | 12 | 292.30±7.66 | 231.31±6.31 | ―60.99±8.99e |
| A* | 12 | 292.23±7.14 | 250.60±7.09 | ―41.63±8.96d |
| B* | 12 | 291.99±6.37 | 261.35±9.04 | ―30.64±8.21c |
| C* | 12 | 292.18±6.63 | 310.97±6.45 | 18.79±10.11a |
| D* | 12 | 292.08±6.64 | 308.48±5.10 | 16.40±7.89a |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download