Abstract
Objectives
We aimed to compare the differences in caries lesion changes when measured by QLF-D as fluorescence loss and by SS-OCT as lesion depth with respect to demineralized time, during formation of artificial early caries lesion. We also demonstrated that QLF-D and SS-OCT can be used effectively in monitoring the longitudinal progression of simulated caries lesions.
Methods
Ten bovine incisors were sectioned (5×4 mm) and embedded in epoxy resin. An acid-resistant nail varnish was applied to a part of the tooth surfaces to protect sound enamel (2×4 mm). To generate lesions, each specimen was immersed in 40 ml of a demineralizing gel for 20 days at 37°C. To measure mineral loss of the demineralized specimens, fluorescence loss (∆F, %) was measured by QLF-D and lesion depth (μm) was determined by SS-OCT from the captured cross-sectional image. All the specimens were analyzed daily by QLF-D image analysis software and SS-OCT image analysis program for 20 days. The repeated measures analysis of ∆F and lesion depth was used. The paired t-test was used to assess differences between each day. The correlation between ∆F and lesion depth was determined using the Pearson’s correlation coefficient.
Results
On the 5th, 10th, and 15th day, compared to baseline values, ∆F decreased in 12.7%, 25.0%, and 33.6% of the specimens, respectively, and the lesion depth increased in 9.9%, 16.0%, and 22.6% of the specimens, respectively. However, after 15 days, there was no change in the ∆F and lesion depth. High significant correlation was identified between the resultant values of ∆F obtained by QLF-D and those of lesion depth obtained by SS-OCT (r = ―0.811, P<0.0001).
Go to : 
References
1. Kim CS, Han SY, Kim CW. The relationship between regional socioeconomic position and oral health behavior: A multilevel approach analysis. J Korean Acad Oral Health. 2013; 37:208–215.

2. Ricketts DN, Kidd EA, Smith BG, Wilson RF. Clinical and radiographic diagnosis of occlusal caries: a study in vitro. J Oral Reha-bil. 1995; 22:15–20.

3. Haak R, Wicht MJ, Hellmich M, Gossmann A, Noack MJ. The validity of proximal caries detection using magnifying visual aids. Caries Res. 2002; 36:249–255.

4. Bader JD, Shugars DA, Bonito AJ. Systematic reviews of selected dental caries diagnostic and management methods. J Dent Educ. 2001; 65:960–968.

6. Gmur R, Giertsen E, van der Veen MH, de Josselin de Jong E, ten Cate JM, Guggenheim B. In vitro quantitative light-induced fluorescence to measure changes in enamel mineralization. Clin Oral Investig. 2006; 10:187–195.
7. Stookey GK. Quantitative light fluorescence: a technology for early monitoring of the caries process. Dent Clin North Am. 2005; 49:753–770. vi.

8. Pretty IA, Pender N, Edgar WM, Higham SM. The in vitro detection of early enamel de- and re-mineralization adjacent to bonded orthodontic cleats using quantitative light-induced fluorescence. Eur J Orthod. 2003; 25:217–223.

9. Ando M, Hall AF, Eckert GJ, Schemehorn BR, Analoui M, Stookey GK. Relative ability of laser fluorescence techniques to quantitate early mineral loss in vitro. Caries Res. 1997; 31:125–131.

10. Gomez J, Tellez M, Pretty I, Ellwood R, Ismail A. Non-cavitated carious lesions detection methods: a systematic review. Community Dent Oral Epidemiol. 2013; 41:55–66.

11. Chew HP, Zakian CM, Pretty IA, Ellwood RP. Measuring initial enamel erosion with quantitative light-induced fluorescence and optical coherence tomography: an in vitro validation study. Caries Res. 2014; 48:254–262.

12. Natsume Y, Nakashima S, Sadr A, Shimada Y, Tagami J, Sumi Y. Estimation of lesion progress in artificial root caries by swept source optical coherence tomography in comparison to transverse microradiography. J Biomed Opt. 2011; 16:071408.

13. Amaechi BT, Podoleanu A, Higham SM, Jackson DA. Correlation of quantitative light-induced fluorescence and optical coherence tomography applied for detection and quantification of early dental caries. J Biomed Opt. 2003; 8:642–647.

14. Jones RS, Darling CL, Featherstone JD, Fried D. Imaging artificial caries on the occlusal surfaces with polarization-sensitive optical coherence tomography. Caries Res. 2006; 40:81–89.

15. Shimada Y, Sadr A, Burrow MF, Tagami J, Ozawa N, Sumi Y. Validation of swept-source optical coherence tomography (SS-OCT) for the diagnosis of occlusal caries. J Dent. 2010; 38:655–665.

16. Nakagawa H, Sadr A, Shimada Y, Tagami J, Sumi Y. Validation of swept source optical coherence tomography (SS-OCT) for the diagnosis of smooth surface caries in vitro. J Dent. 2013; 41:80–89.

17. Lim H, de Boer JF, Park BH, Lee EC, Yelin R, Yun SH. Optical frequency domain imaging with a rapidly swept laser in the 815-870 nm range. Opt Express. 2006; 14:5937–5944.

18. Chinn SR, Swanson EA, Fujimoto JG. Optical coherence tomography using a frequency-tunable optical source. Opt Lett. 1997; 22:340–342.

19. Fried D, Xie J, Shafi S, Featherstone JD, Breunig TM, Le C. Imaging caries lesions and lesion progression with polarization sensitive optical coherence tomography. J Biomed Opt. 2002; 7:618–627.

20. Gomez J, Zakian C, Salsone S, Pinto SC, Taylor A, Pretty IA, et al. In vitro performance of different methods in detecting occlusal caries lesions. J Dent. 2013; 41:180–186.

21. Pretty IA, Ingram GS, Agalamanyi EA, Edgar WM, Higham SM. The use of fluorescein-enhanced quantitative light-induced fluorescence to monitor de- and re-mineralization of in vitro root caries. J Oral Rehabil. 2003; 30:1151–1156.

22. Alammari MR, Smith PW, de Josselin de Jong E, Higham SM. Quantitative light-induced fluorescence (QLF): a tool for early occlusal dental caries detection and supporting decision making in vivo. J Dent. 2013; 41:127–132.

23. Hariri I, Sadr A, Shimada Y, Tagami J, Sumi Y. Effects of structural orientation of enamel and dentine on light attenuation and local refractive index: an optical coherence tomography study. J Dent. 2012; 40:387–396.

24. Stamnes JJ, Sithambaranathan GS. Reflection and refraction of an arbitrary electromagnetic wave at a plane interface separating an isotropic and a biaxial medium. J Opt Soc Am A Opt Image Sci Vis. 2001; 18:3119–3129.

25. Kim HE, Kwon HK, Kim BI. Recovery percentage of remineralization according to severity of early caries. Am J Dent. 2013; 26:132–136.
Go to : 
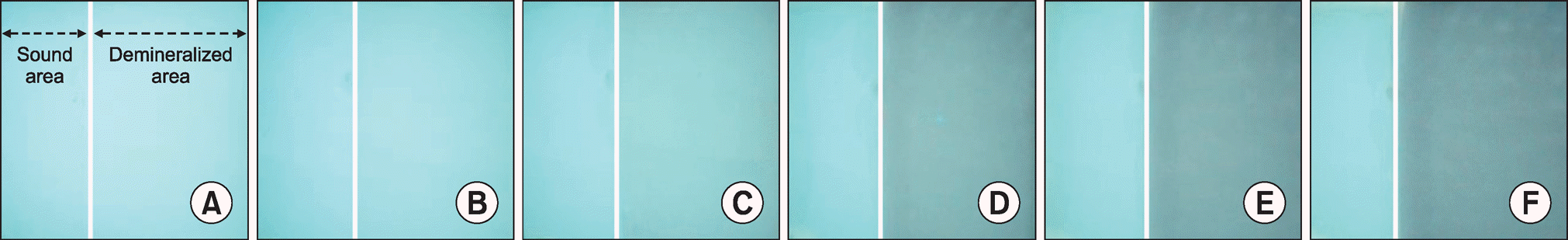 | Fig. 3.Images taken by blue light of QLF-D over demineralization time. (A) base line, (B) day 1, (C) day 5, (D) day 10, (E) day 15, and (F) day 20. In the image, a left arrow indicates the sound area and a right arrow shows the demineralized area of the photographed specimen. |
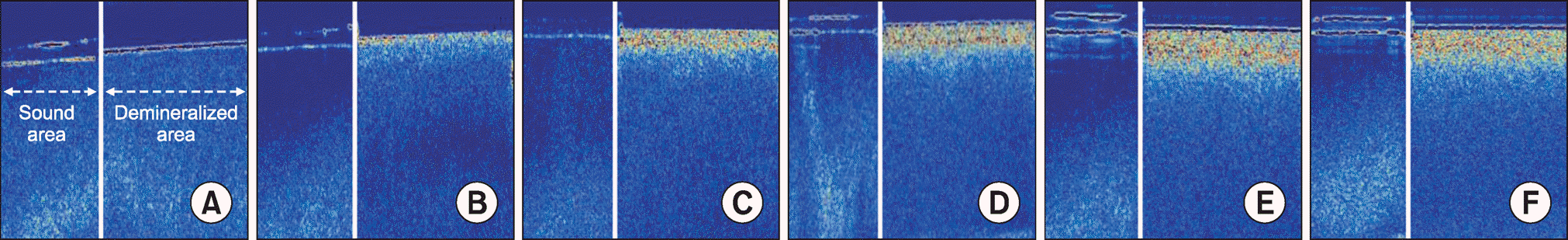 | Fig. 4.Images taken by SS-OCT over demineralization time. (A) base line, (B) day 1, (C) day 5, (D) day 10, (E) day 15, and (F) day 20. In the image, a left arrow indicates the sound area and a right arrow shows the demineralized area of the scanned specimen. |
Table 1.
Changes in fluorescence and lesion depth by demineralization time
All values are the mean (standard deviation). ∆F means the loss of fluorescence in demineralized enamel part compared to sound part (%). LD means the lesion depth measured in images taken by SS-OCT (mm). *P-values denote statistically significant differences within rows by repeated measures analysis. †P-values denote statistically significant differences by paired t-test.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download