Abstract
Objectives
The aim of this study was to examine the effect of a Polycan-calcium gluconate complex on gingival health.
Methods
Forty-one subjects with mild periodontitis (≥40 years) were divided into two groups: the placebo and test product (Polycan-calcium gluconate complex twice a day for 4 weeks) groups. Oral examination was performed and gingival crevicular fluid (GCF) was collected from each subject at baseline and after 4 weeks. Interleukin (IL)-1β level in the GCF was determined using enzyme-linked immunosorbent assay.
Go to : 
References
2. Ministry of Health and Welfare. 2011 National Health Insurance Statistical Yearbook. Seoul: Ministry of Health & Welfare;2011. p. 555–617.
3. Lee SY, Jung YH, Kim KH, Yang BK, Han SB, Chung CP, et al. Matrix metalloproteinases and tissue inhibitors of matrix metalloproteinases in gingival crevicular fluids of periodontitis patients. J Korean Acad periodontol. 2004; 34:139–148.

4. Dongari-Bagtzoglou AI, Ebersole JL. Production of inflammatory mediators and cytokines by human gingival fibroblasts following bacterial challenge. J Periodontal Res. 1996; 31:90–98.

5. Birkedal-Hansen H. Role of cytokine and inflammatory mediators in tissue destruction. J Periodontal Res. 1993; 28:500–510.
6. Hernández Ríos M, Sorsa T, Obregón F, Tervahartiala T, Valenzuela MA, Pozo P, et al. Proteolytic roles of matrix metalloproteinase (MMP)-13 during progression of chronic periodontitis: initial evidence for MMP-13/MMP-9 activation cascade. J Clin Periodontol. 2009; 36:1011–1017.

8. Khoo JG, Newman HN. Subgingival plaque control by a simplified oral hygiene regime plus local chlorhexidine or metronidazole. J Periodontal Res. 1983; 18:607–619.

9. Chan GCF, Chan WK, Sze DMY. The effects of β-glucan on human immune and cancer cells. J Hematol Oncol. 2009; 2:1–11.

10. Song HS, Moon KY. In vitro antioxidant activity profiles of β-glucan isolated from yeast Saccharomayces cerevisiae and mutant Saccharomayces cerevisiae IS2. Food Sci Biotechnol. 2006; 15:437–440.
11. Kogan G, Pajtinka M, Babioncova M, Miadokova E, Rauko P, Sla-menova D, et al. Yeast cell wall polysaccharides as antioxidants and antimutagens: can they fight cancer? Neoplasma. 2008; 55:387–393.
12. Novak M, Vetvicka V. Beta-glucans, history, and the present: immunomodulatory aspects and mechanisms of action. J Immuno-toxicol. 2008; 5:47–57.
13. Abdel-Salam OM, Baiuomy AR, El-batran S, Arbid MS. Evaluation of the anti-inflammatory, anti-nociceptive and gastric effects of Ginkgo biloba in the rat. Pharmacol Res. 2004; 49:133–142.

14. Shin HD, Yang KJ, Park BR, Son CW, Jang HJ, Ku SK. Antios-teoporotic effect of polycan, beta-glucan from Aureobasidium, in ovariectomized osteoporotic mice. Nutrition. 2007; 23:853–860.
15. Song HB, Park DC, Do GM, Hwang SL, Lee WK, Kang HS, et al. Effect of exopolymers of Aureobasidium pullulans on improving osteoporosis induced in ovariectomized mice. J Microbiol Biotechnol. 2006; 16:37–45.
16. Queiroz LS, Nascimento MS, Cruz AK, Castro AJ, Moura Mde F, Baseia IG, et al. Glucans from the Caripia montagnei mushroom present anti-inflammatory activity. Int Immunopharmacol. 2010; 10:34–42.

17. Kim YS, Kang SJ, Kim JW, Cho HR, Moon SB, Kim KY, et al. Effects of Polycan, a β-glucan, on experimental periodontitis and alveolar bone loss in Sprague-Dawley rats. J Periodontal Res. 2012; 47:800–810.

18. Bracken WM, Cuppage F, McLaury RL, Kirwin C, Klaassen CD. Comparative effectiveness of topical treatments for hydrofluoric acid burns. J Occup Med. 1985; 27:733–739.
19. Cavallini M, de Boccard F, Corsi MM, Fassati LR, Baruffaldi Preis FW. Serum pro-inflammatory cytokines and chemical acid burns in rats. Ann Burns Fire Disasters. 2004; 17:84–87.
20. Karnad AS, Patil PA, Majagi SI. Calcium enhances anti-inflammatory activity of aspirin in albino rats. Indian J Pharmacol. 2006; 38:397–402.

21. Ku SK, Cho HR, Sung YS, Kang SJ, Lee YJ. Effects of calcium gluconate on experimental periodontitis and alveolar bone loss in rats. Basic Clin Pharmacol Toxicol. 2011; 108:241–250.

22. Silness J, Löe H. Periodontal disease in pregnancy.II. Correlation between oral hygiene and periodontal condition. Acta Odontol Scand. 1964; 22:121–135.
23. Ainslie PT, Caffesse RG. A biometric evaluation of gingival curettage (II). Quintessence Int Dent Dig. 1981; 12:609–614.
24. Cercek JF, Kiger RD, Garrett S, Egelberg J. Relative effects of plaque control and instrumentation on the clinical parameters of human periodontal disease. J Clin Periodontol. 1983; 10:46–56.

25. Figueredo CM, Ribeiro MS, Fischer RG, Gustafsson A. Increased interleukin-1β concentration in gingival crevicular fluid as a characteristic of periodontitis. J Periodontol. 1999; 70:1457–1463.

26. Gamonal J, Acevedo A, Bascones A, Jorge O, Silva A. Level of interleukin-1β, -8, and -10 and RANTES in gingival crevicular fluid and cell populations in adult periodontitis patients and the effect of periodontal treatment. J Periodontol. 2000; 71:1535–1545.
Go to : 
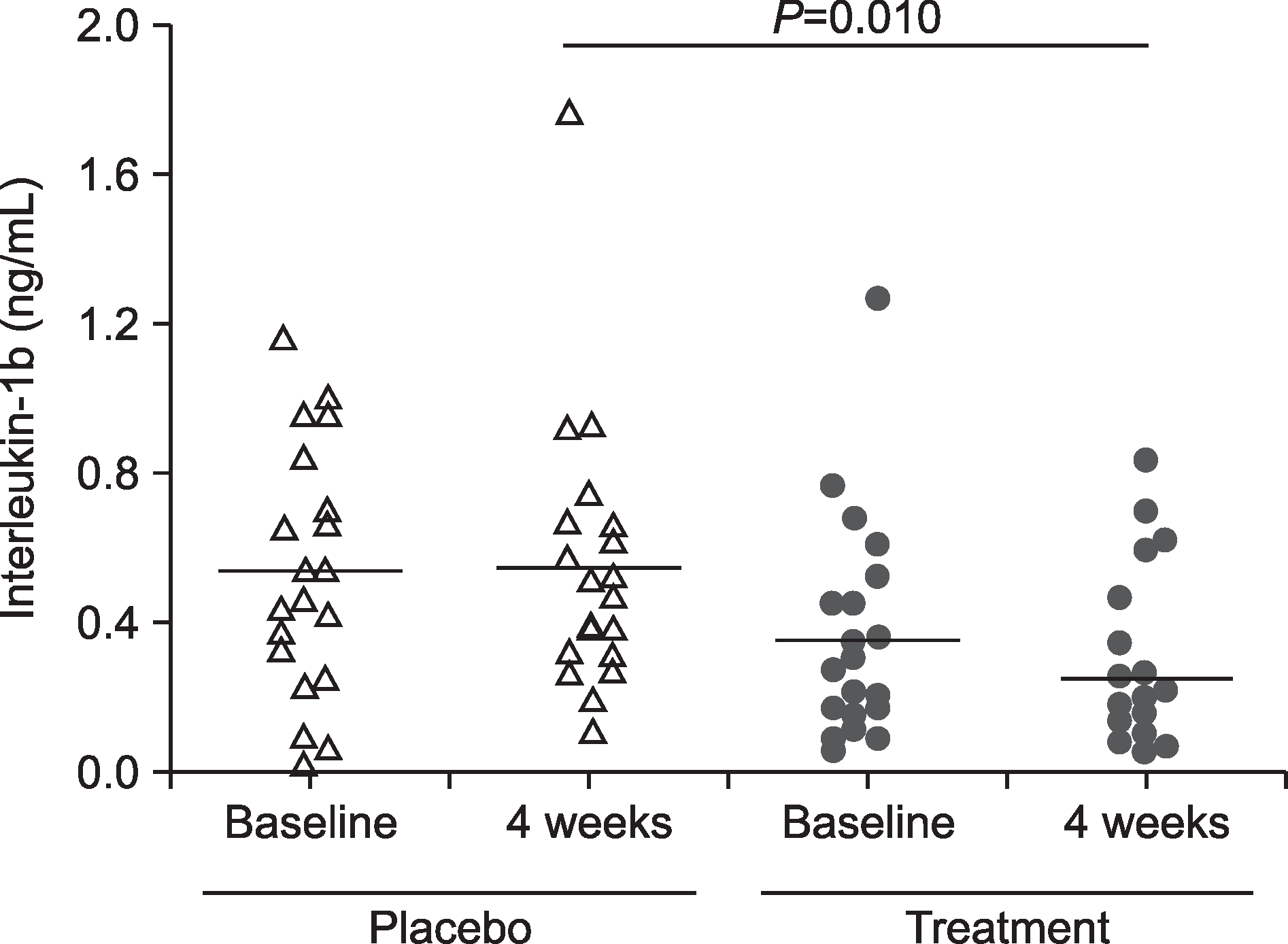 | Fig. 1.Comparison of IL-1β in the gingival crevicular fluid between the two groups by ELISA. Triangle and circle indicate IL-1β level of each subject. P-value obtained from Mann-Whitney test. |
Table 1.
General characteristics of study subject
Table 2.
Oral health characteristics of study subject
Table 3.
Mean changes of clinical parameters according to times by groups clinical parameters in the control and treatment groups at baseline, 4 weeks
| Placebo (N=21) | Treatment (N=20) | |||||
|---|---|---|---|---|---|---|
| Baseline | After 4 weeks | P-value* | Baseline | After 4 weeks | P-value* | |
| Pocket depth (mm) | 1.58±0.31 | 1.47±0.34 | 0.008 | 1.58±0.31 | 1.48±0.28 | 0.035 |
| Clinical attachment loss (mm) | 0.45±0.45 | 0.44±0.44 | 0.814 | 0.37±0.35 | 0.37±0.34 | 0.677 |
| Gingival index | 1.30±0.38 | 1.19±0.28 | 0.201 | 1.20±0.31 | 1.06±0.29 | 0.058 |
| Plaque index | 1.46±0.43 | 1.33±0.31 | 0.139 | 1.41±0.45 | 1.12±0.34† | 0.015 |
| Bleeding index | 5.33±4.56 | 3.62±2.91 | 0.072 | 3.90±2.90 | 2.85±2.30 | 0.065 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download