Abstract
Objectives
The purpose of this study was to evaluate the bleaching effect of chewing gum containing amorphous calcium phosphate (ACP), hydroxyapatite (HA), and tricalcium pyrophosphate (TSP) on human enamel.
Methods
Seventy-three subjects aged 20-30 years were recruited after obtaining their informed consent and approval of the Institutional Review Board. All subjects were randomly assigned to the following four groups: (I) negative control group; (II) 50% gum group; (III) 100% gum group; and (IV) positive control group (10% carbamide peroxide). They received gum containing ACP, HA, and TSP, three times a day, for 4 weeks. Group IV also received 10% CP application using individual trays, once a day, for 2 weeks. Color change was measured using the Shade Eye-NCC colorimeter at weekly intervals, during the 4-week period. Statistical analysis was performed using SPSS 18.0.
Results
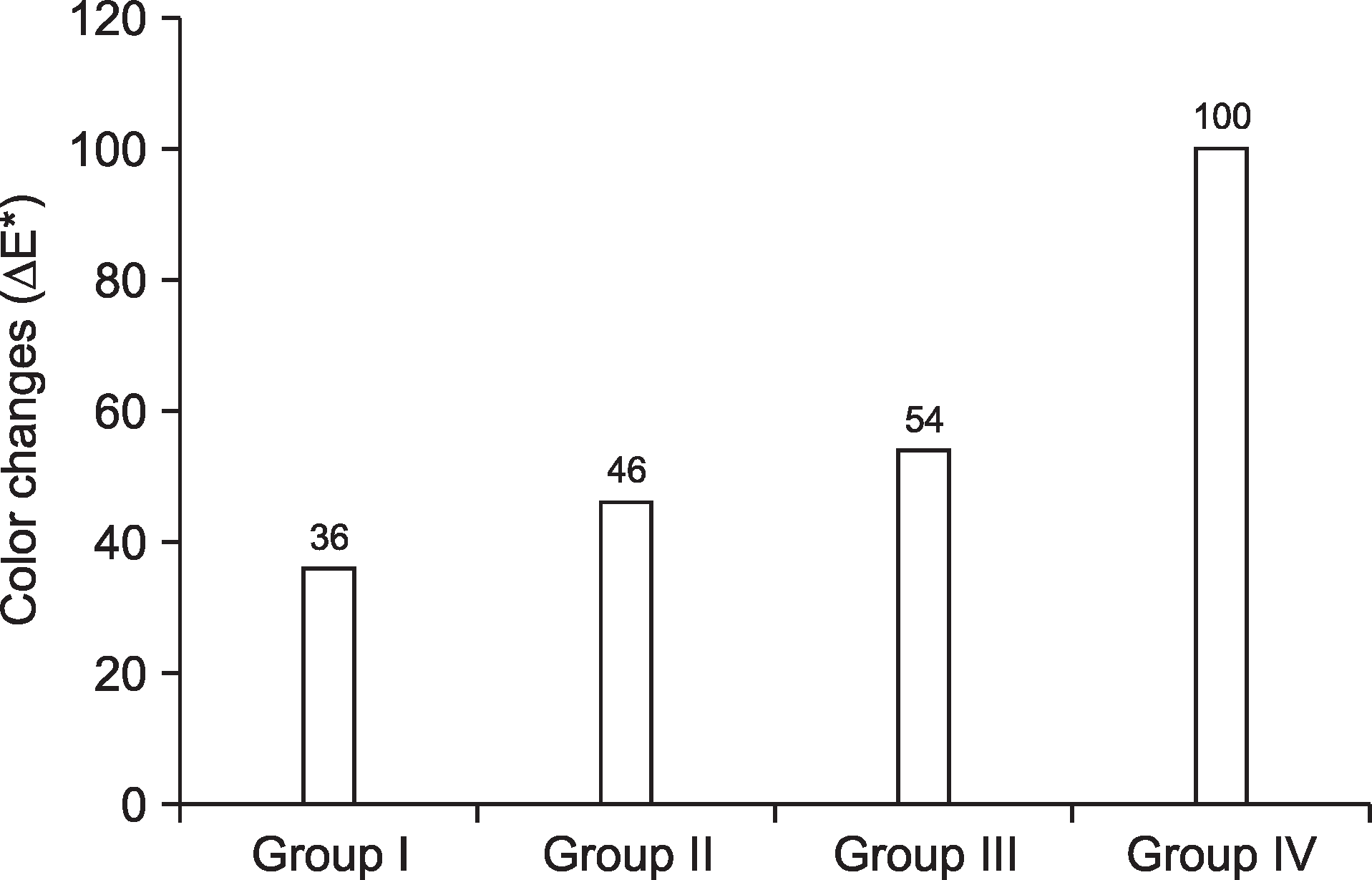
Color changes (∆E*) were significantly different among the groups at 2 and 4 weeks after chewing the gum (P<0.05). Given that bleaching effect of Group IV was 100%, bleaching effects of Group III, Group II, and Group I were 54%, 46%, and 36%, respectively.
Conclusions
Chewing gum containing ACP, HA, and TSP was effective enough to bleach the human enamel. Further comprehensive studies and assessment will be required to ascertain the bleaching effects and mechanism of chewing gum containing various components such as ACP, HA, and TSP using various methods of experiment and analysis.
References
1. Sulieman MA. An overview of tooth-bleaching techniques: chemistry, safety and efficacy. Periodontol 2000. 2008; 48:148–169.
2. Ritter AV, Leonard RH, St Georges AJ, Caplan DJ, Haywood VB. Safety and stability of nightguard vital bleaching: 9 to 12 years post-treatment. J Esthet Restor Dent. 2002; 14:275–285.

3. Saeki Y, Kato T, Okuda K. Inhibitory effects of funoran on the adherence and colonization of oral bacteria. Bull Tokyo Dent Coll. 1996; 37:77–92.
4. Park DY, Ma DS, Cho KM, Jeong DB, Jung SH. Efficacy of a paint-on, self-applied, humidity-facilitated setting 2.8% hydrogen peroxide whitening gel. J Korean Acad Oral Health. 2005; 29:397–406.
5. Jung SH, Park DY, Ma DS, Kim JY, Kim JH. Effect of 2.6% hydrogen peroxide containing tooth - whitening strip on stained hydroxyapatite tablets. J Korean Acad Oral Health. 2002; 26:375–383.
6. Lee SY, Jeong SH, Kang SM, Kwon HK, Kim BI. Effect of 2.8% hydrogen peroxide gel on tooth whitening in vitro. J Korean Acad Oral Health. 2007; 31:20–30.
7. Jung SH, Park DY, Ma DS. Efficacy of six months of a 2.6% hydrogen peroxide containing tooth-whitening strips. J Korean Acad Oral Health. 2004; 28:117–125.
8. Jung SH, Bae KH, Lee WJ, Moon HS, Paik DI, Kim JB. Clinical study on the effect of therapeutic toothpaste containing tetrasodium pyrophosphate, vitamin E and hydrogen peroxide on the tooth whitening. J Korean Acad Oral Health. 2001; 25:221–226.
9. Porciani PF, Grandini S, Perra C, Grandini R. Whitening effect by stain inhibition from a chewing gum with sodium hexametaphos-phate in a controlled twelve-week single-blind trial. J Clin Dent. 2006; 17:14–16.
10. DE Abreu DR, Sasaki RT, Amaral FL, Flório FM, Basting RT. Effect of home-use and in-office bleaching agents containing hydrogen peroxide associated with amorphous calcium phosphate on enamel microhardness and surface roughness. J Esthet Restor Dent. 2011; 23:158–168.

11. Grobler SR, Majeed A, Moola MH, Rossouw RJ, van Wyk Kotze T. In vivo spectrophotometric assessment of the tooth whitening effectiveness of nite white 10% with amorphous calcium phosphate, potassium nitrate and fluoride, over a 6-month period. Open Dent J. 2011; 5:18–23.
12. Pimenta LA. Commentary. Effect of home-use and in-office bleaching agents containing hydrogen peroxide associated with amorphous calcium phosphate on enamel microhardness and surface roughness. J Esthet Restor Dent. 2011; 23:169–170.

13. Gu HJ, Lee YE, Baek HJ, Kim JS, Song KB. Effect of fluoride application after tooth bleaching using the diode Laser. J Korean Acad Oral Health. 2008; 32:160–169.
14. Borges BC, Pinheiro MH, Feitosa DA, Correia TC, Braz R, Montes MA, et al. Preliminary study of a novel in-office bleaching therapy modified with a casein phosphopeptide-amorphous calcium phosphate. Microsc Res Tech. 2012; 75:1571–1575.

15. Browning WD, Cho SD, Deschepper EJ. Effect of a nano-hydroxyapatite paste on bleaching-related tooth sensitivity. J Esthet Restor Dent. 2012; 24:268–276.

16. Lobene RR. A clinical study of the anticalculus effect of a dentifrice containing soluble pyrophosphate and sodium fluoride. Clin Prev Dent. 1986; 8:5–7.
17. Kim JB, Paik DI, Moon HS, Ma DS. A clinical comparison of the anticalculus effect of two kinds of therapeutic dentifrice. J Korean Acad Oral Health. 1989; 13:7–15.
18. Moon HS, Jin BH, Park DY, Ma DS, Paik DI, Kim JB, et al. A clinical study of toothpaste containing triclosan, pyrophosphate and tranexamic acid on the prevention of calculus formation and gingivitis. J Korean Acad Oral Health. 1994; 18:545–553.
19. Moon HS, Jung SH, Paik DI, Kim JB, Yoon JW, Shin MJ, et al. The effect of dentifrice containing triclosan, pyrophosphate and tranexamic acid on the antimicrobial activity against the pathogens of periodontal disease and halitosis. J Korean Acad Oral Health. 1998; 22:205–214.
20. Schemehorn BR, Moore MH, Putt MS. Abrasion, polishing, and stain removal characteristics of various commercial dentifrices in vitro. J Clin Dent. 2011; 22:11–18.
21. Farrell S, Barker ML, Gerlach RW, Putt MS, Milleman JL. Prevention of lingual calculus formation with daily use of 6% H2O2/2% pyrophosphate whitening strips. J Clin Dent. 2009; 20:75–78.
22. Lenhard M. Assessing tooth color change after repeated bleaching in vitro with a 10 percent carbamide peroxide gel. J Am Dent Assoc. 1996; 127:1618–1624.
Table 1
Table 2.
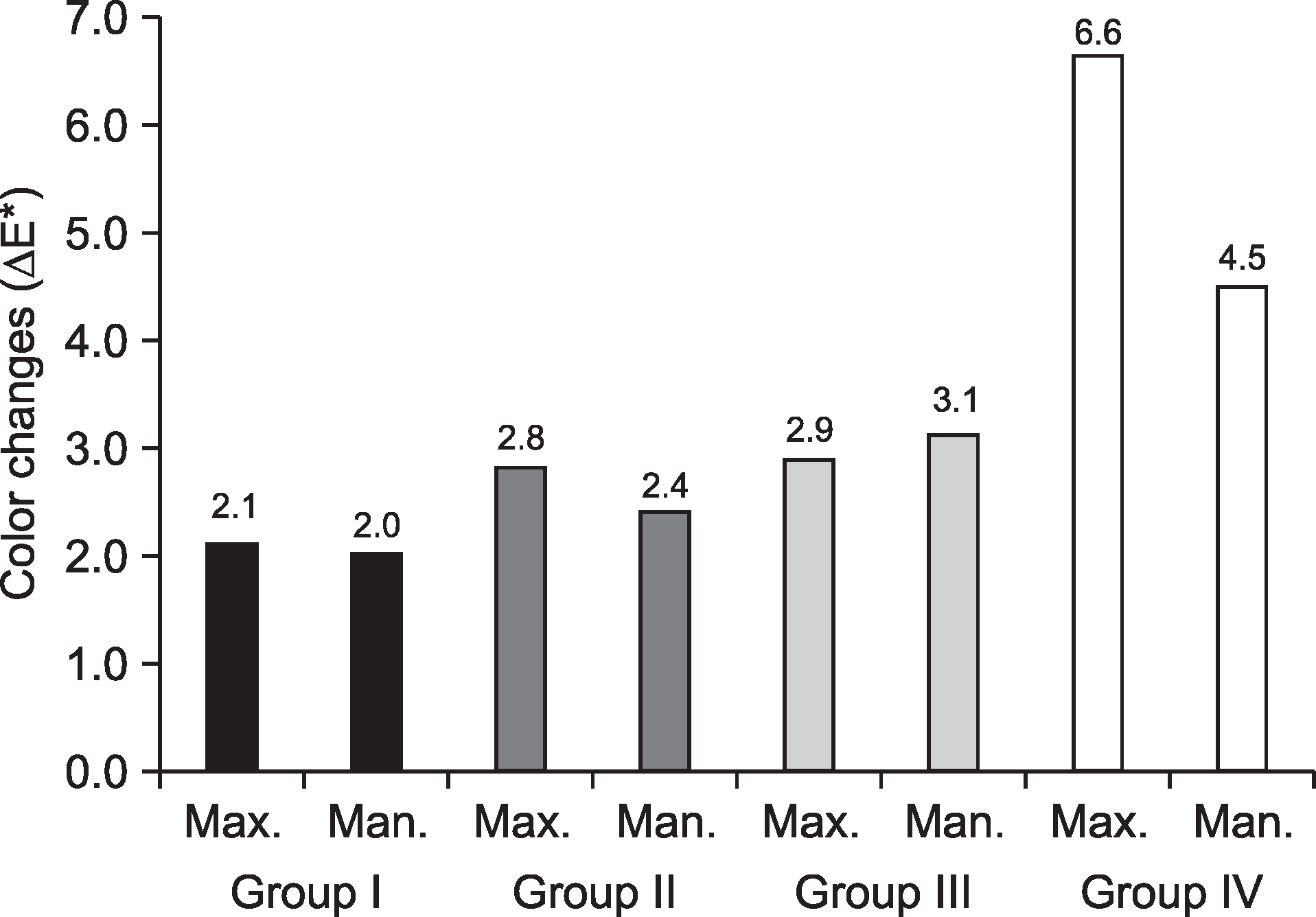
Color changes (ΔE*) of all teeth by bleaching times
| Group | 1 week | 2 weeks* | 3 weeks | 4 weeks* |
|---|---|---|---|---|
| I† | 1.71±0.92 | 1.43±0.84a | 0.95±0.72 | 2.04±0.90a |
| II† | 1.82±1.30 | 1.69±1.14ab | 2.33±1.16 | 2.59±1.74ab |
| III† | 2.04±1.29 | 2.24±1.64b | 3.88±6.48 | 2.98±1.86b |
| IV | - | 5.52±2.04 | - | - |
| P-value | <0.017 | <0.032 |
Table 3.
Color changes of specimens with time according to the NBS system




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download