Abstract
Alpha-fetoprotein (AFP) is frequently used for hepatocellular carcinoma (HCC) diagnosis and surveillance. Although the current ARCHITECT AFP (List number 7K67) assay range is 0–350 ng/mL, all samples with test results between 200 and 350 ng/mL must be diluted and retested until their levels are <200 ng/mL. A new ARCHITECT AFP (8100/3P36) assay with a dynamic range of up to 2000 ng/mL has been introduced. The aim of this study was to perform a method comparison between the current ARCHITECT AFP assay and the new assay. The precision study showed excellent results for both high and low controls. There was a positive correlation between the two assay systems and clinical samples. The new ARCHITECT AFP assay with a wide assay range demonstrated good analytical performance. Therefore, the current ARCHITECT AFP assay could be replaced by the new assay, which is more convenient and minimizes manual labor.
Hepatocellular carcinoma (HCC) is the most common primary hepatic malignancy and HCC incidence has been increasing recently in the United States [1234]. In Korea, hepatic neoplasm is the fifth most common cancer and HCC comprises up to 75% of all hepatic neoplasms [56]. The 5-year survival rate of HCC patients is low, approximately 23.3% from 2004 to 2008, indicating a significantly poor prognosis compared to other cancers in Korea [5]. Because many HCCs are asymptomatic prior to the development of end stage disease, regular HCC surveillance is mandatory for patients with chronic hepatitis or cirrhosis to enable tumor detection at an early stage and to improve patient outcome following curative treatment [7]. Therefore, early diagnosis and early detection of tumor recurrence or disease progression are the most important factors for improving prognosis. Alpha-fetoprotein (AFP) was first introduced as a serological marker for HCC. To date, most practice guidelines recommend routine surveillance for HCC, using ultrasonography and serum tumor markers including AFP, both for diagnosis and as an indicator of tumor response to treatment [8910111213].
Although the assay range of the current ARCHITECT AFP (List number 7K67) is 0–350 ng/mL, all samples with test results between 200 and 350 ng/mL must be diluted and retested until their levels are <200 ng/mL. This leads to an increase in the number of bioanalytical samples, necessitating the development of higher throughput methods to cope with the increased workload. Therefore, a new reagent has been introduced for high-throughput and its configuration, diluent formulation, and manufacturing process have been redesigned. The new ARCHITECT AFP (8100/3P36) assay was intended to have a dynamic range of up to 2,000 ng/mL. In this study, we aimed to evaluate the new ARCHITECT AFP (8100/3P36) assay. We assessed the performance of the new assay and performed a method comparison between the current ARCHITECT AFP assay and the new assay. Our study included 150 samples from HCC patients and 69 samples from healthy individuals for whom AFP tests had been requested. Sera were separated immediately after arrival and all residual samples were stored at −70℃ until the AFP assay was conducted. AFP was measured using a chemiluminescent microparticle immunoassay (CMIA) with an Abbott Architect analyzer (Abbott Laboratories, Abbott Park, IL, USA) and both the current and new reagent. The precision (coefficient of variation, CV) and linearity of the new AFP assay were assessed. The assay precision of the new AFP test was evaluated according to CLSI protocol EP5-A2 [14]. AFP concentration was measured twice daily for five consecutive days using the low and high level control materials (6.87 ng/mL and 1,998.43 ng/mL, respectively). The linearity of the new AFP assay was validated based on CLSI protocol EP6-A [15]. Samples with five levels were prepared by mixing the high and low level pooled patient sera (15.0 ng/mL and 2,000.0 ng/mL, respectively) and were tested in duplicate. AFP concentrations determined with the new AFP assay were compared with those measured using the current AFP assay. All statistical analyses were performed using MedCalc version 9.3 (MedCalc Software, Mariakerke, Belgium). The new AFP assay was compared with the current AFP assay by calculating the Pearson's correlation coefficient. Data were also analyzed using Analyse-it (Analyse-it Software Ltd., Leeds, UK). P values <0.05 were regarded as statistically significant.
Of the total 219 samples, 150 samples were from HCC patients. The median age of the patients was 61 years (range: 31–85) and 114 (767%) were male. The median age of the 69 healthy individuals was 49 (range: 27–63) and 25 (36.2%) were male. The median AFP levels of patients and healthy individuals determined using the current and new AFP assay were 5.12 (range: 0.81–1,447.11) and 4.62 (0.58–1,661.46); and 2.51 (1.16–8.13) and 2.15 (0.84–8.10), respectively. High AFP levels (>200 ng/mL) were observed in six samples using the current AFP assay; these also exhibited high AFP levels using the new AFP assay without the dilution procedure. The results of the precision study showed that the total precisions (CV) were excellent (<5%). Between-day precision was 3.1% with the low control and 3.8% with the high control and total precision was 3.7% (low control) and 4.1% (high control), respectively (Table 1). The new AFP assay showed good linearity for the expected values (y=1.0021x–22.253, r2=0.9994; Fig. 1). In addition, there was excellent correlation between the results using the new and current AFP reagent with patient sera (r=0.8522, P<0.0001) and with sera from healthy individuals (r=0.9574, P<0.0001; Fig. 2). The Bland-Altman mean bias was 1.92 (upper limit, 21.34; lower limit, −18.96) and 0.34 (1.07, −0.39) for results from patients and healthy individuals, respectively.
In addition to the frequent use of AFP for HCC diagnosis and surveillance, several recent studies have reported that changes in AFP level following treatment may serve as a marker of response to treatment [1516]. Therefore, an accurate and simple method is required for AFP testing. However, the current AFP assay with a lower dynamic range is relatively labor-intensive. This report is a comparison study using a new AFP assay for HCC in patients and healthy individuals. Some of the results showed differences between the current AFP assay and the new assay; however, these differences were not considered clinically different. We conclude that the new AFP assay exhibits excellent correlation with the current AFP assay. In addition, the assay range was expanded from 200 ng/mL to 2,000 ng/mL. The new AFP assay can also be set up to auto-dilute samples that have results >2,000 ng/mL (1:10 dilution). This assay improvement dramatically reduces the requirement to retest samples with high AFP levels. In terms of precision analysis, the new AFP assay was reported to show good analytical performance concordant with the current study, with <5% CV for all assessed samples [16]. Therefore, we anticipate that the turnaround time for AFP testing could be shortened with reduced hands-on time, providing a rapid test without the need for any other instruments.
This study has some limitations. We could not obtain samples from newly diagnosed HCC patients and we compared samples with relatively lower AFP levels. In addition, the smaller population in our study and reference group selection may have influenced the results. Nevertheless, our study demonstrates that the new AFP assay has an improved dynamic range, excellent assay performance, and excellent correlation with the current AFP assay.
In conclusion, the current ARCHITECT AFP assay could be replaced by a new assay that is more convenient and minimizes manual labor. Further studies with different patient groups or different settings and monitoring AFP levels following treatment are necessary to demonstrate the prognostic ability and usefulness of AFP in patients with HCC.
Figures and Tables
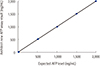 | Fig. 1Linearity analysis of new ARCHITECT AFP assay. The new AFP assay showed good linearity for the expected values (y=1.0021x–22.253, r2=0.9994). |
 | Fig. 2Comparison of new and current ARCHITECT AFP assay in patients (A) and healthy individuals (B). There was excellent correlation between the results of the new and current assays in both patients (r=0.8522, P<0.0001) and healthy individuals (r=0.9574, P<0.0001). |
References
1. El-Serag HB. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999; 340:745–750.

3. But DY, Lai CL, Yuen MF. Natural history of hepatitis-related hepatocellular carcinoma. World J Gastroenterol. 2008; 14:1652–1656.

4. Davila JA, Henderson L, Kramer JR, Kanwal F, Richardson PA, Duan Z, et al. Utilization of surveillance for hepatocellular carcinoma among hepatitis C virus-infected veterans in the United States. Ann Intern Med. 2011; 154:85–93.

5. Ministry of Health and Welfare. 2008 Annual Report of Cancer Statistics in Korea. Gwacheon: Ministry of Heath and Welfare;2010.
6. Ministry of Heath and Welfare. Cancer Incidence in Korea 1999–2001. Gwacheon: Ministry of Heath and Welfare;2005.
7. Park JW. National policy of early detection in hepatocellular carcinoma. Korean J Hepatol. 2002; 8:S16.
8. Chalasani N, Said A, Ness R, Hoen H, Lumeng L. Screening for hepatocellular carcinoma in patients with cirrhosis in the United States: results of a national survey. Am J Gastroenterol. 1999; 94:2224–2229.

10. Zhang BH, Yang BH, Tang ZY. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol. 2004; 130:417–422.

11. Riaz A, Ryu RK, Kulik LM, Mulcahy MF, Lewandowski RJ, Minocha J, et al. Alpha-fetoprotein response after locoregional therapy for hepatocellular carcinoma: oncologic marker of radiologic response, progression, and survival. J Clin Oncol. 2009; 27:5734–5742.

12. Vora SR, Zheng H, Stadler ZK, Fuchs CS, Zhu AX. Serum alpha-fetoprotein response as a surrogate for clinical outcome in patients receiving systemic therapy for advanced hepatocellular carcinoma. Oncologist. 2009; 14:717–725.

13. Chan SL, Mo FK, Johnson PJ, Hui EP, Ma BB, Ho WM, et al. New utility of an old marker: serial α-fetoprotein measurement in predicting radiologic response and survival of patients with hepatocellular carcinoma undergoing systemic chemotherapy. J Clin Oncol. 2009; 27:446–452.

14. National Committee for Clinical Laboratory Standards. Evaluation of precision performance of quantitative measurement methods; approved standard. EP5-A2. Wayne, PA: National Committee for Clinical Laboratory Standards;2004.
15. National Committee for Clinical Laboratory Standards. Evaluation of the linearity of quantitative measurement procedures: a statistical approach: approved guideline. EP6-A. Wayne, PA: National Committee for Clinical Laboratory Standards;2003.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download