Abstract
Background:
Natural killer (NK) cells play a key role in innate immune responses and are an important component of anti-cancer defenses. This study aimed to investigate the clinicopathological characteristics of NK cell activity (NKA) among various hematological malignancies at diagnosis and to evaluate their clinical value as a monitoring marker.
Methods:
A total of 111 patients that were newly diagnosed with hematological malignancies were recruited, comprising 18 acute myeloid leukemia (AML), 31 multiple myeloma (MM), and 62 lymphoma. Twenty-three normal control subjects from our health examination center were recruited. NKA was measured using a commercially available enzyme-linked immunosorbent assay kit, which measures interferon-gamma secreted by ex vivo-stimulated NK cells in whole blood.
Results:
The 111 patients had a median NKA of 202.80 pg/mL (range 40–2,000). NKA was significantly decreased in patients with AML (median 47.05 pg/mL, 40–2,000, P<0.0001), MM (275.00, 40–2,000, P<0.0001), and lymphoma (289.49, 40–2,000, P<0.0001) compared with that in normal controls (1,891, 412–2,000). There was a difference in NKA between AML and lymphoma (P=0.0499). Serial changes in NKA correlated with disease progression. NKA did not correlate with the NK cell count in any group of hematological malignancies.
Go to : 
REFERENCES
1.Campbell KS., Hasegawa J. Natural killer cell biology: an update and future directions. J Allergy Clin Immunol. 2013. 132:536–44.

2.Benjamin JE., Gill S., Negrin RS. Biology and clinical effects of natural killer cells in allogeneic transplantation. Curr Opin Oncol. 2010. 22:130–7.

3.Hazeldine J., Lord JM. The impact of ageing on natural killer cell function and potential consequences for health in older adults. Ageing Res Rev. 2013. 12:1069–78.

4.Huang H., Patel DD., Manton KG. The immune system in aging: roles of cytokines, T cells and NK cells. Front Biosci. 2005. 10:192–215.

5.Imai K., Matsuyama S., Miyake S., Suga K., Nakachi K. Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: an 11-year follow-up study of a general population. Lancet. 2000. 356:1795–9.

6.Schleypen JS., Von Geldern M., Weiss EH., Kotzias N., Rohrmann K., Schendel DJ, et al. Renal cell carcinoma-in trating natural killer cells express differential repertoires of activating and inhibitory receptors and are inhibited by speci HLA class I allotypes. Int J Cancer. 2003. 106:905–12.
7.Mandal A., Viswanathan C. Natural killer cells: in health and disease. Hematol Oncol Stem Cell Ther. 2015. 8:47–55.

8.Ruggeri L., Mancusi A., Burchielli E., Aversa F., Martelli MF., Velardi A. Natural killer cell alloreactivity in allogeneic hematopoietic transplantation. Curr Opin Oncol. 2007. 19:142–7.

9.Baier C., Fino A., Sanchez C., Farnault L., Rihet P., Kahn-Perles B, et al. Natural killer cells modulation in hematological malignancies. Front Immunol. 2013. 4:459.

10.Viel S., Charrier E., Marcais A., Rouzaire P., Bienvenu J., Karlin L, et al. Monitoring NK cell activity in patients with hematological malignancies. Oncoimmunology. 2013. 2:e26011.

11.Miller JS. Therapeutic applications: natural killer cells in the clinic. Hematology Am Soc Hematol Educ Program. 2013. 2013:247–53.

12.Rezvani K., Rouce RH. The application of natural killer cell immunotherapy for the treatment of cancer. Front Immunol. 2015. 6:578.

13.Lee SB., Cha J., Kim IK., Yoon JC., Lee HJ., Park SW, et al. A high-through put assay of NK cell activity in whole blood and its clinical application. Biochem Biophys Res Commun. 2014. 445:584–90.
14.Koo KC., Shim DH., Yang CM., Lee SB., Kim SM., Shin TY, et al. Reduction of the CD16(-)CD56bright NK cell subset precedes NK cell dysfunction in prostate cancer. PLoS One. 2013. 8:e78049.

15.Cheson BD., Bennett JM., Kopecky KJ., Buchner T., Willman CL., Estey EH, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003. 21:4642–9.

16.Palumbo A., Rajkumar SV., San Miguel JF., Larocca A., Niesvizky R., Morgan G, et al. International Myeloma Working Group consensus statement for the management, treatment, and supportive care of patients with myeloma not eligible for standard autologous stem-cell transplantation. J Clin Oncol. 2014. 32:587–600.

17.Cheson BD., Pfstner B., Juweid ME., Gascoyne RD., Specht L., Horning SJ, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007. 25:579–86.

18.Mukaka MM. Statistics corner: a guide to appropriate use of correlation coeffcient in medical research. Malawi Med J. 2012. 24:69–71.
19.Whiteside TL., Friberg D. Natural killer cells and natural killer cell activity in chronic fatigue syndrome. Am J Med. 1998. 105:27S–34S.

20.Farnault L., Sanchez C., Baier C., Le Treut T., Costello RT. Hematological malignancies escape from NK cell innate immune surveillance: mechanisms and therapeutic implications. Clin Dev Immunol. 2012. 2012:421702.

21.Stringaris K., Sekine T., Khoder A., Alsuliman A., Razzaghi B., Sargeant R, et al. Leukemia-induced phenotypic and functional defects in natural killer cells predict failure to achieve remission in acute myeloid leukemia. Haematologica. 2014. 99:836–47.

22.Parry HM., Stevens T., Oldreive C., Zadran B., McSkeane T., Rudzki Z, et al. NK cell function is markedly impaired in patients with chronic lymphocytic leukaemia but is preserved in patients with small lymphocytic lymphoma. Oncotarget. 2016. 7:68513–26.

23.Konjevic G., Jurisic V., Banicevic B., Spuzic I. The difference in NK-cell activity between patients with non-Hodgkin's lymphomas and Hodgkin's disease. Br J Haematol. 1999. 104:144–51.
24.Brenner CD., King S., Przewoznik M., Wolters I., Adam C., Bornkamm GW, et al. Requirements for control of B-cell lymphoma by NK cells. Eur J Immunol. 2010. 40:494–504.

25.Khaznadar Z., Boissel N., Agaugue S., Henry G., Cheok M., Vignon M, et al. Defective NK cells in acute myeloid leukemia patients at diagnosis are associated with blast transcriptional signatures of immune evasion. J Immunol. 2015. 195:2580–90.

26.Jurisic V., Srdic T., Konjevic G., Markovic O., Colovic M. Clinical stage-depending decrease of NK cell activity in multiple myeloma patients. Med Oncol. 2007. 24:312–7.

27.Mitsiades CS., Chen-Kiang S. Immunomodulation as a therapeutic strategy in the treatment of multiple myeloma. Crit Rev Oncol Hematol. 2013. 88(Suppl 1):S5–13.

28.Dunbar EM., Buzzeo MP., Levine JB., Schold JD., Meier-Kriesche HU., Reddy V. The relationship between circulating natural killer cells after reduced intensity conditioning hematopoietic stem cell transplantation and relapse-free survival and graft-versus-host disease. Haematologica. 2008. 93:1852–8.

Go to : 
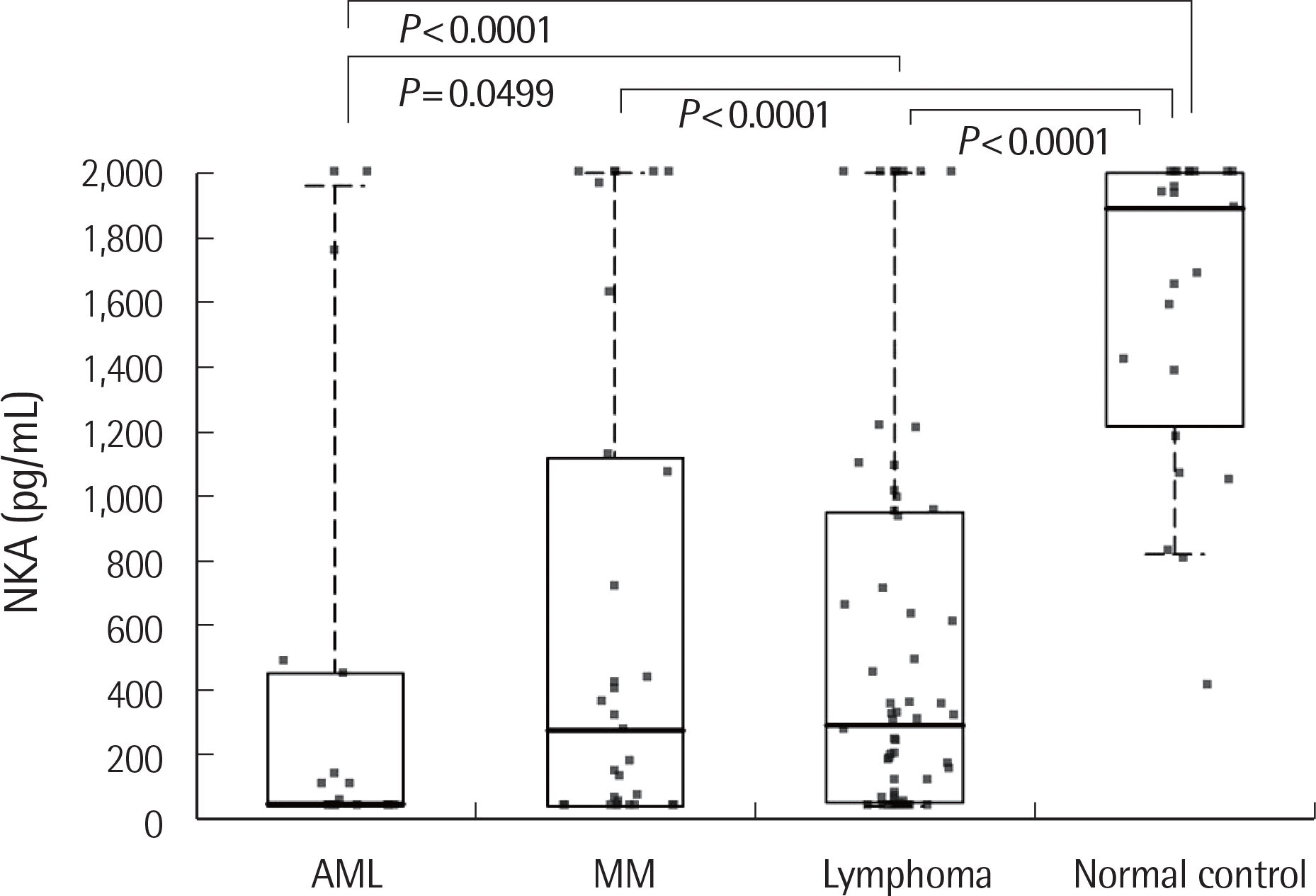 | Fig. 1.Comparisons of the natural killer (NK) cell activity (NKA) among patients with hematological malignancies at diagnosis and controls. Boxes are inter-quartile ranges, horizontal bars are medians, and dotted lines are 10th and 90th percentiles. |
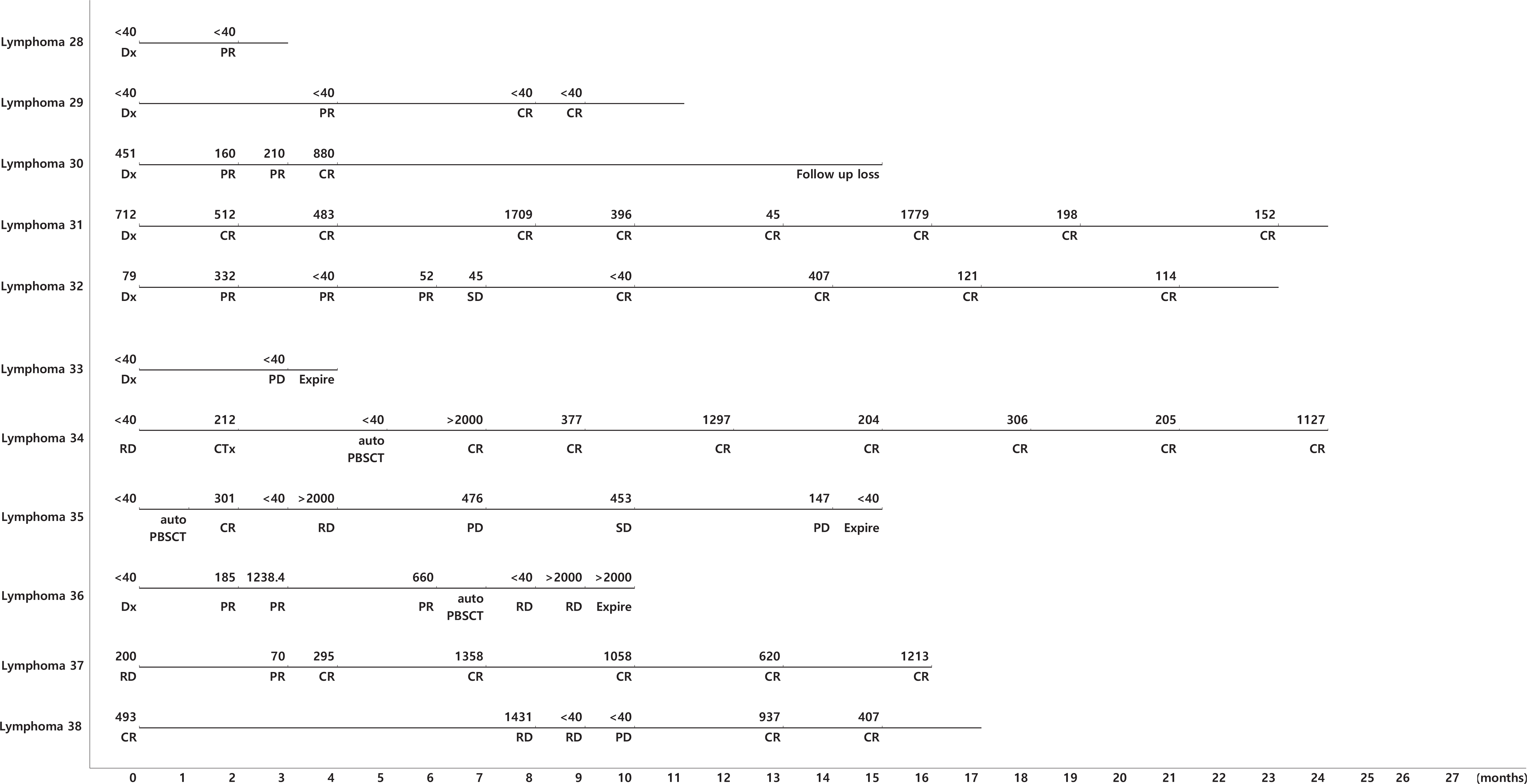 | Fig. 2.Serial analysis of natural killer (NK) cell activity (NKA) during follow-up in patients with hematological malignancies. NKA is presented as pg/mL. Abbreviations: Allo-PBSCT, allogeneic peripheral blood stem cell transplantation; Auto-PBSCT, autologous peripheral blood stem cell transplantation; CR, complete response; Dx, diagnosis; PD, progressive disease; PR, partial response; RD, relapsed disease; SD, stable disease. |
Table 1.
Demographic and laboratory features of patients with hematological malignancies at diagnosis
| AML (N=18) | MM (N=31) | Lymphoma (N=62) | Normal Control (N=23) | P | |
|---|---|---|---|---|---|
| Age (year) | 56.5 (34–92) | 70.0 (40–89) | 59.5 (17–90) | 51.0 (37–59) | <0.0001 |
| Sex ratio (Male:Female) | 1.57:1 | 1.38:1 | 0.82:1 | 0.53:1 | 0.2317 |
| WBC (/µL) | 3,225 (770–133,710) | 5,740 (1,470–13,550) | 6,585 (1,840–21,000) | 4,950 (2,590–8,940) | 0.0930 |
| Neutrophil count (/µL) | 480 (0–11,524) | 3,796 (610–8,640) | 3,874 (930–10,650) | 2,690 (1,060–3,800) | 0.0001 |
| Lymphocyte count (/µL) | 1,137 (540–19,114) | 1,539 (310–6,998) | 1,698 (190–19,320) | 2,052 (1,128–4,657) | 0.0756 |
| Neutrophil / Lymphocyte (ratio) | 0.31 (0.00–6.00) | 2.17 (0.10–17.79) | 2.49 (0.06–92.95) | 1.24 (0.67–2.93) | <0.0001 |
| Lymphocyte percentage (%) | 41.2 (8.0–83.1) | 30.5 (7.1–87.8) | 25.9 (2.2–92.0) | 37.6 (24.0–52.1) | 0.0007 |
| NK cell activity (pg/mL)∗ | 47.05 (40–2,000) | 275.00 (40–2,000) | 289.49 (40–2,000) | 1,891.00 (412–2,000) | 0.1595 |
| NK cell (CD3-/CD56+/16+) percentage (%) | 11.7 (2.5–35.6) | 18.9 (5.3–66.2) | 15.2 (2.3–48.2) | NT | 0.1276 |
| NK cell (CD3-/CD56+/16+) count (/µL) | 206 (31–4,102) | 317 (48–1,489) | 222 (9–1,503) | NT | 0.2127 |
| CD3+ cell count (/µL) | 1,195 (461–10,464) | 1,144 (215–6,353) | 1,114 (142–3,818) | NT | 0.5369 |
| CD3+/CD4+ cell count (/µL) | 820 (261–5,857) | 550 (95–5,891) | 571 (55–2,148) | NT | 0.2753 |
| CD3+/CD8+ cell count (/µL) | 441 (106–4,136) | 384 (7–1,436) | 325 (54–1,574) | NT | 0.4294 |
| CD19+ cell count (/µL) | 169 (2–4,266) | 129 (11–436) | 161 (6–13,955) | NT | 0.6061 |
| CRP (mg/dL) | 2.00 (0.08–25.61) | 0.81 (0.04–9.64) | 0.51 (0.04–15.19) | NT | 0.0985 |
Table 2.
Correlations of NK cell activity with WBC parameters including NK cell numbers and lymphocyte subsets in patients with hematological malignancies at diagnosis




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download