Abstract
Background:
Whole blood viscosity (WBV) refers to the internal resistance that occurs when blood flows through blood vessels. WBV is known to be related to many diseases including cardiovascular and neurovascular diseases. We have investigated the analytical performance and established reference intervals for a newly developed microfluidic viscometer, Viscore-300 (NanoBiz, Korea), used for the measurement of WBV.
Methods:
We performed a precision test of 240 measurements over 20 days using three control materials. For evaluation of repeatability, a total of 60 WBV measurements were made in 3 whole blood samples 20 times a day. A total of 100 whole blood samples were used to evaluate the accuracy of the Viscore-300 in comparison to a rotating viscometer, DV3T (Brookfield, USA), in accordance with the the Clinical and Laboratory Standards Institute's guidelines. To establish the reference intervals, 122 healthy individuals were enrolled in this study.
Results:
The precision and repeatability results showed that the CV was less than 5% for three samples and two shear rates. In the accuracy test, the mean differences between two viscometers were 0.09 cP (0.9%) and –0.07 cP (–1.4%) at shear rates of 10 s–1 and 300 s–1, respectively. The reference intervals of WBV for men were 6.88–13.52 cP at 10 s–1 and 4.32–6.43 cP at 300 s–1; those of women were 5.74–13.29 cP at 10 s–1 and 3.60–6.12 cP at 300 s–1.
Go to : 
REFERENCES
1.Junker R., Heinrich J., Ulbrich H., Schulte H., Rainer Schönfeld R., Köhler E, et al. Relationship between plasma viscosity and the severity of coronary heart disease. Arterioscler Thromb Vasc Biol. 1998. 18:870–5.

2.Fossum E., Hoieggen A., Moan A., Nordby G., Velund TL., Kjeldsen SE. Whole blood viscosity, blood pressure and cardiovascular risk factors in healthy blood donors. Blood Press. 1997. 6:161–5.

3.Song SH., Kim JH., Lee JH., Yun YM., Choi DH., Kim HY. Elevated blood viscosity is associated with cerebral small vessel disease in patients with acute ischemic stroke. BMC Neurol. 2017. 17:20.

4.Rosencranz R., Bogen SA. Clinical laboratory measurement of serum, plasma, and blood viscosity. Am J Clin Pathol. 2006. 125:S78–86.

5.Clinical and Laboratory Standards Institute. Evaluation of precision performance of quantitative measurement methods; approved guide-line—2nd ed. CLSI document EP05-A3. Wayne, PA: Clinical and Laboratory Standards Institute. 2014.
6.Clinical and Laboratory Standards Institute. Measurement procedure comparison and bias estimation using patient samples; approved guide-line—3rd ed. CLSI document EP09-A3. Wayne, PA: Clinical and Laboratory Standards Institute. 2013.
7.Kim H., Cho YI., Lee DH., Park CM., Moon HW., Hur M, et al. Analytical performance evaluation of the scanning capillary tube viscometer for measurement of whole blood viscosity. Clin Biochem. 2013. 46:139–42.

8.Jung JM., Lee DH., Kim KT., Choi MS., Cho YG., Lee HS, et al. Reference intervals for whole blood viscosity using the analytical performance-evaluated scanning capillary tube viscometer. Clin Biochem. 2014. 47:489–93.

9.Clinical and Laboratory Standards Institute. Defning, establishing, and verifying reference intervals in the clinical laboratory; approved guideline—3rd ed. CLSI document EP28-A3c. Wayne, PA: Clinical and Laboratory Standards Institute. 2008.
10.R Core Team. R: a language and environment for statistical computing [Internet]. Vienna, Austria: R Foundation for Statistical Computing, 2016. Available from:. https://www.R-project.org/.
11.Froom P. Blood viscosity and the risk of death from coronary heart disease. Eur Heart J. 2000. 21:513–4.
12.Minato S., Takenouchi A., Uchida J., Tsuboi A., Kurata M., Fukko K, et al. Association of whole blood viscosity with metabolic syndrome in type 2 diabetic patients: independent association with post-breakfast tri-glyceridemia. J Clin Med Res. 2017. 9:332–8.

13.Kang YJ., Yoon SY., Lee KH., Yang S. A highly accurate and consistent microfuidic viscometer for continuous blood viscosity measurement. Artif Organs. 2010. 34:944–9.
14.Kang YJ., Yang S. Integrated microfuidic viscometer equipped with fuid temperature controller for measurement of viscosity in complex fuids. Microfuid Nanofuidics. 2013. 14:657–68.
Go to : 
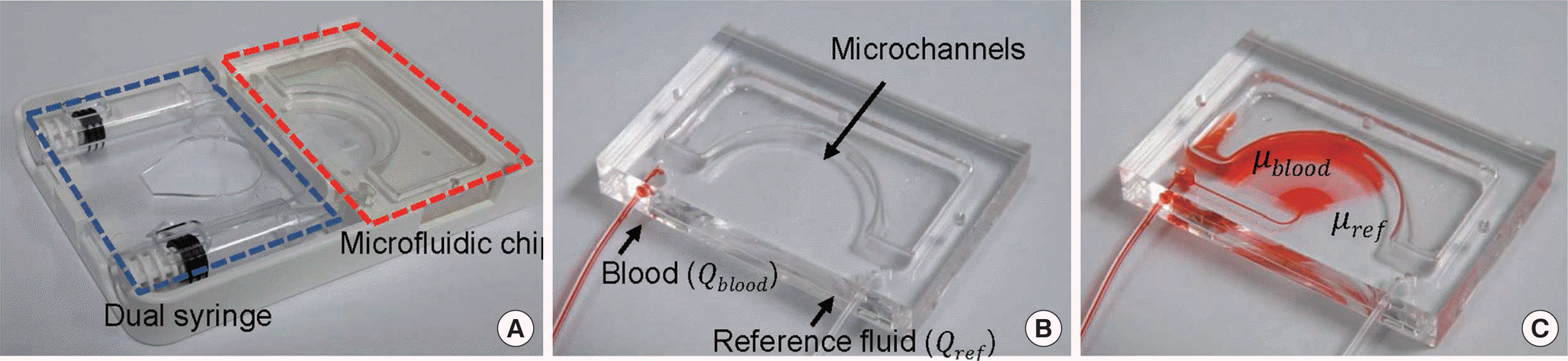 | Fig. 1.The core chip and measurement processes of Viscore. (A) The microfluidic chip (V-Chip) and dual syringes for whole blood and reference fluid are mounted in a disposable cartridge (a photograph of a finished product). (B) Initial step of fluid injection (an experiment photograph of V-Chip). (C) Late step to measure whole blood viscosity (an experiment photograph of V-Chip). The number of microchannels filled with whole blood and reference fluid are directly counted, and viscosity is determined accordingly. |
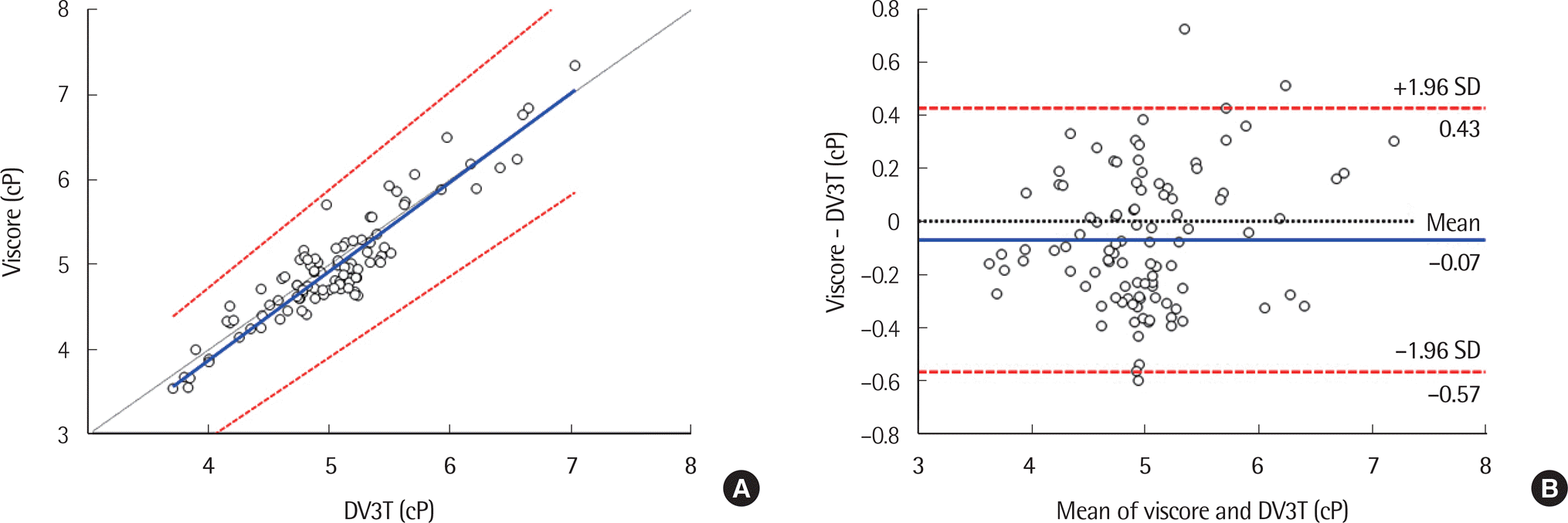 | Fig. 2.Results of accuracy test using 100 whole blood samples at a high shear rate, 300 s-1, between DV3T and Viscore. (A) Passing-Bablok regression plot. X-axis represents the viscosity measured by DV3T as a reference method. Y-axis represents the viscosities measured by the Viscore, as a comparative method. The black dash line is an identity line (y=x), the blue solid line is a regression line, and the shade area presents 95% confidence interval of the Passing-Bablok regression. (B) Bland-Altman difference plot. X-axis represents the average viscosity measured by DV3T and Viscore as a reference method. Y-axis represents the difference of viscosities between Viscore and DV3T. The black dash line is a base line (difference=0), the blue solid line is a mean difference between the two viscometers, and the red dash lines presents ±1.96 SD of mean bias. 3 samples were out of ±1.96 SD of mean bias. |
Table 1.
Precision of Viscore-300 using three control materials at shear rates of 10 and 300 s–1 (total 240 tests)
Table 2.
Repeatability of Viscose-300 using whole blood samples at shear rates of 10 and 300 s–1 (total 60 tests)
| Specimen concentration | Shear rate (s-1) | Average viscosity (cP) | Coefficient of variance, CV (%) |
|---|---|---|---|
| Low | 10 | 6.98 | 4.21 |
| 300 | 3.36 | 3.34 | |
| Medium | 10 | 9.45 | 3.88 |
| 300 | 4.54 | 2.90 | |
| High | 10 | 12.68 | 4.80 |
| 300 | 5.16 | 3.61 |
Table 3.
Results of WBV measured by Viscore-300 and DV3T
Table 4.
Parameters of Passing-Bablok regression line between Vis-core-300 and DV3T
| Shear rate (s–1) | Parameter of regression | Values (95% CI) |
|---|---|---|
| 10 | Intercept | 0.08 (–0.43–0.54) |
| Slope | 1.00 (0.95–1.05) | |
| R | 0.974 | |
| 300 | Intercept | –0.33 (–0.85–0.11) |
| Slope | 1.05 (0.95–1.15) | |
| R | 0.925 |
Table 5.
The characteristics and hematological profiles of healthy individuals for determination of reference interval
| Variable | Total | Men (N=82) | Women | P∗ |
|---|---|---|---|---|
| (N=122) | (N=40) | |||
| Age (year) | 36.5±9.5† | 37.8±9.1† | 34.0±10.1† | 0.042 |
| White blood cell (×103/µL) | 6.6±1.8 | 6.8±1.8 | 6.0±1.7 | 0.022 |
| Red blood cell (×103/µL) | 4.8±0.4 | 5.0±0.3 | 4.5±0.2 | <0.001 |
| Hemoglobin (g/dL) | 14.8±1.5 | 15.6±0.9 | 13.2±1.3 | <0.001 |
| Hematocrit (%) | 43.0±3.7 | 44.8±2.4 | 39.0±3.0 | <0.001 |
| Platelets (×103/µL) | 258.0±54.6 | 252.4±50.2 | 269.9±62.1 | 0.104 |
Table 6.
Reference intervals (2.5th–97.5th percentiles) of human whole blood viscosity measured by Viscore-300
| Shear rate (s-1) | Group | Median WBV (cP) | Reference Intervals∗ (cP) |
|---|---|---|---|
| 10 | Total (N=122) | 9.46 | 6.55–13.40 |
| Men (N=82) | 9.95 | 6.88–13.52 | |
| Women (N=40) | 8.39 | 5.74–13.29 | |
| 300 | Total (N=122) | 4.96 | 3.76–6.26 |
| Men (N=82) | 5.15 | 4.32–6.43 | |
| Women (N=40) | 4.50 | 3.60–6.12 |
∗ Healthy individuals were selected as those without any cardiovascular disease, neurovascular disease, liver disease, diabetes, dyslipidemia, and history of medication. Non-parametric estimation was used to establish reference intervals for WBV. Abbreviations: cP, centipoise; WBV, whole blood viscosity.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download