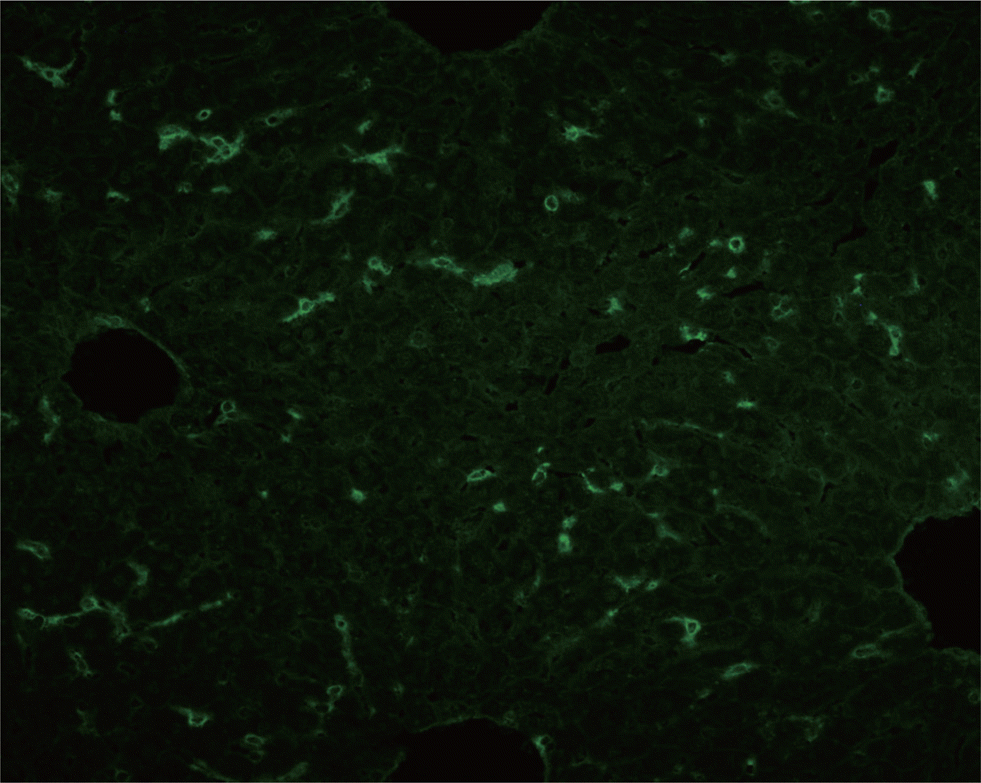
Abstract
Bile canalicular antibody (BCA) was first reported in 1969. Many studies of BCA were performed in the 1970s and 1980s and revealed that BCA has a highly positive rate in chronic active hepatitis and primary biliary cirrhosis (PBC). These studies suggested that BCA can be useful in the diagnosis of these liver diseases. However, BCA is almost negative in patients with alcoholic hepatitis. We report a case of BCA in a 50-yr-old woman with a history of heavy alcohol consumption. The patient's serum levels of aspartate transaminase and alanine transaminase were increased, leading to a diagnosis of alcoholic hepatitis. The patient was evaluated for liver disease. Anti-mitochondria antibody, anti-smooth muscle antibody, and anti-liver kidney microsomal antibody tests were conducted, yielding negative results. However, during this testing process, the patient's serum was incidentally found to be positive for BCA at a titer of 1:160. This is the first case report of BCA in Korea.
REFERENCES
1. Diederichsen H. Hetero-antibody against bile canaliculi in patients with chronic, clinically active hepatitis. Acta Med Scand. 1969; 186:299–302.

2. MacSween RN, Armstrong EM, Gray KG, Mason M. Bile canalicular antibody in primary biliary cirrhosis and in other liver diseases. Lancet. 1973; 1:1419–21.

3. Kurki P, Miettinen A, Linder E, Pikkarainen P, Vuoristo M, Salaspuro MP. Different types of smooth muscle antibodies in chronic active hepatitis and primary biliary cirrhosis: their diagnostic and prognostic signifcance. Gut. 1980; 21:878–84.
4. Diederichsen H, Riisom K, Andersen I, Hage E. Antibodies to isolated hepatocytes in chronic active liver disease. Identifcation and diagnostic signifcance of bile canalicular antibodies. Scand J Gastroenterol. 1986; 21:146–50.
5. Fischer JT, Petz LD, Garratty G, Cooper NR. Correlations between quantitative assay of red cell-bound C3, serologic reactions, and hemolytic anemia. Blood. 1974; 44:359–73.

6. Tuma DJ, Klassen LW. Immune responses to acetaldehyde-protein adducts: Role in alcoholic liver disease. Gastroenterology. 1992; 103:1969–73.

8. Jara P, Hierro L, Martínez-Fernández P, Alvarez-Doforno R, Yánez F, Diaz MC, et al. Recurrence of bile salt export pump defciency after liver transplantation. N Engl J Med. 2009; 361:1359–67.
9. Pauli-Magnus C, Meier PJ. Hepatocellular transporters and cholestasis. J Clin Gastroenterol. 2005; 39:S103–10.

10. Borst P, de Wolf C, van de Wetering K. Multidrug resistance-associated proteins 3, 4, and 5. Pfugers Arch. 2007; 453:661–73.

11. Smit JJ, Schinkel AH, Oude Elferink RP, Groen AK, Wagenaar E, van Deemter L, et al. Homozygous disruption of the murine mdr2 P-glycoprotein gene leads to a complete absence of phospholipid from bile and to liver disease. Cell. 1993; 75:451–62.

13. Dunn W, Angulo P, Sanderson S, Jamil LH, Stadheim L, Rosen C, et al. Utility of a new model to diagnose an alcohol basis for steatohepatitis. Gastroenterology. 2006; 131:1057–63.

14. Altamirano J, Miquel R, Katoonizadeh A, Abraldes JG, Duarte-Rojo A, Louvet A, et al. A histologic scoring system for prognosis of patients with alcoholic hepatitis. Gastroenterology. 2014; 146:1231–9.
15. Naveau S, Giraud V, Borotto E, Aubert A, Capron F, Chaput JC. Excess weight risk factor for alcoholic liver disease. Hepatology. 1997; 25:108–11.

16. Carithers RL Jr, Herlong HF, Diehl AM, Shaw EW, Combes B, Fallon HJ, et al. Methylprednisolone therapy in patients with severe alcoholic hepatitis. A randomized multicenter trial. Ann Intern Med. 1989; 110:685–90.
17. Dunn W, Jamil LH, Brown LS, Wiesner RH, Kim WR, Menon KV, et al. MELD accurately predicts mortality in patients with alcoholic hepatitis. Hepatology. 2005; 41:353–8.

18. Dominguez M, Rincón D, Abraldes JG, Miquel R, Colmenero J, Bellot P, et al. A new scoring system for prognostic stratifcation of patients with alcoholic hepatitis. Am J Gastroenterol. 2008; 103:2747–56.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download