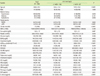
1. Hong KS, Bang OY, Kang DW, Yu KH, Bae HJ, Lee JS, et al. Stroke statistics in Korea: part I. Epidemiology and risk factors: a report from the korean stroke society and clinical research center for stroke. J Stroke. 2013; 15:2–20.

2. Sherwood MW, Kristin Newby LK. High-sensitivity troponin assays: evidence, indications, and reasonable use. J Am Heart Assoc. 2014; 3:e000403.

3. Kim SJ, Moon GJ, Bang OY. Biomarkers for stroke. J Stroke. 2013; 15:27–37.

4. Kerr G, Ray G, Wu O, Stott DJ, Langhorne P. Elevated troponin after stroke: a systematic review. Cerebrovasc Dis. 2009; 28:220–226.

5. Di Angelantonio E, Fiorelli M, Toni D, Sacchetti ML, Lorenzano S, Falcou A, et al. Prognostic significance of admission levels of troponin I in patients with acute ischaemic stroke. J Neurol Neurosurg Psychiatry. 2005; 76:76–81.

6. Song HS, Back JH, Jin DK, Chung PW, Moon HS, Suh BC, et al. Cardiac troponin T elevation after stroke: relationships between elevated serum troponin T, stroke location, and prognosis. J Clin Neurol. 2008; 4:75–83.

7. Faiz KW, Thommessen B, Einvik G, Omland T, Rønning OM. Prognostic value of high-sensitivity cardiac troponin T in acute ischemic stroke. J Stroke Cerebrovasc Dis. 2014; 23:241–248.

8. Barber M, Morton JJ, Macfarlane PW, Barlow N, Roditi G, Stott DJ. Elevated troponin levels are associated with sympathoadrenal activation in acute ischemic stroke. Cerebrovasc Dis. 2007; 23:260–266.

9. Etgen T, Baum H, Sander K, Sander D. Cardiac troponins and N-terminal pro-brain natriuretic peptide in acute ischemic stroke do not relate to clinical prognosis. Stroke. 2005; 36:270–275.

10. Jensen JK, Ueland T, Aukrust P, Antonsen L, Kristensen SR, Januzzi JL, et al. Highly sensitive troponin T in patients with acute ischemic stroke. Eur Neurol. 2012; 68:287–293.

11. Saenger AK, Beyrau R, Braun S, Cooray R, Dolci A, Freidank H, et al. Multicenter analytical evaluation of a high-sensitivity troponin T assay. Clin Chim Acta. 2011; 412:748–754.

12. Anders B, Alonso A, Artemis D, Schäfer A, Ebert A, Kablau M, et al. What does elevated high-sensitive troponin I in stroke patients mean: concomitant acute myocardial infarction or a marker for high-risk patients? Cerebrovasc Dis. 2013; 36:211–217.

13. Král M, Šaňák D, Veverka T, Hutyra M, Vindiš D, Kunčarová A, et al. Troponin T in acute ischemic stroke. Am J Cardiol. 2013; 112:117–121.

14. Stahrenberg R, Niehaus CF, Edelmann F, Mende M, Wohlfahrt J, Wasser K, et al. High-sensitivity troponin assay improves prediction of cardiovascular risk in patients with cerebral ischaemia. J Neurol Neurosurg Psychiatry. 2013; 84:479–487.

15. Jensen JK, Atar D, Mickley H. Mechanism of troponin elevations in patients with acute ischemic stroke. Am J Cardiol. 2007; 99:867–870.

16. Jensen JK, Kristensen SR, Bak S, Atar D, Høilund-Carlsen PF, Mickley H. Frequency and significance of troponin T elevation in acute ischemic stroke. Am J Cardiol. 2007; 99:108–112.

17. Christensen H, Johannesen HH, Christensen AF, Bendtzen K, Boysen G. Serum cardiac troponin I in acute stroke is related to serum cortisol and TNF-alpha. Cerebrovasc Dis. 2004; 18:194–199.

18. Jespersen CM, Fischer Hansen J. Myocardial stress in patients with acute cerebrovascular events. Cardiology. 2008; 110:123–128.

19. Folsom AR, Nambi V, Bell EJ, Oluleye OW, Gottesman RF, Lutsey PL, et al. Troponin T, N-terminal pro-B-type natriuretic peptide, and incidence of stroke: the atherosclerosis risk in communities study. Stroke. 2013; 44:961–967.

20. Oluleye OW, Folsom AR, Nambi V, Lutsey PL, Ballantyne CM. ARIC Study Investigators. Troponin T. B-type natriuretic peptide, C-reactive protein, and cause-specific mortality. Ann Epidemiol. 2013; 23:66–73.







 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download