Abstract
Although neutrophilia can manifest from various causes, it is important to be able to distinguish chronic neutrophilic leukemia (CNL) from neutrophilic leukemoid reactions (NLR). In this paper, we describe four cases of leukocytosis with neutrophilia, including one case of CNL with a T618I mutation in colony stimulating factor 3 receptor (CSF3R) and three cases of NLR associated with malignancy or sepsis, which were initially suspected as CNL. Of the three NLR cases, one was associated with ovarian cancer, one with monoclonal gammopathy of undetermined significance and one with multiple myeloma with sepsis. This study demonstrated that confirming the clonality of myeloid cells with CSF3R T618I could contribute to making an accurate differential diagnosis between CNL and NLR in patients with solid cancers or plasma cell neoplasms caused by paraneoplastic syndromes and/or infection.
Neutrophilia can occur due to chronic neutrophilic leukemia (CNL) or neutrophilic leukemoid reaction (NLR) associated with infection, neoplasm, inflammation, drug use, and acute hemorrhaging, among other causes [12]. CNL is a subtype of myeloproliferative neoplasm associated with a mutation in the colony stimulating factor 3 receptor (CSF3R) gene [3]. The protein encoded by this gene is the receptor for colony stimulating factor 3 that produces a signal through downstream SRC family kinases and Janus kinase (JAK) pathways and plays an important role in the growth and differentiation of granulocytes [4]. There are two different classes of CSF3R mutation: One is a truncation mutation, which results in dysregulation of downstream SRC family kinases and leads to constitutive overexpression of the receptor and ligand hypersensitivity. The other is a membrane proximal mutation which results in dysregulation of the JAK family and constitutive activation of the receptor in the absence of granulocyte-colony stimulating factor (G-CSF) ligand [4]. An example of the membrane proximal mutation is on exon 14 of CSF3R resulting in T618I mutation, which has been identified in more than 80% of CNL cases [3].
CSF3R T618I could be used as a molecular marker to make a differential diagnosis between CNL and NLR. It has been reported that NLR can develop in 10% of patients with solid tumors (lung, genitourinary, gastrointestinal, bone metastases, breast cancer, etc.) [12], and may be due to the paraneoplastic production of G-CSF or other growth factors. Furthermore, NLR can be present years before the diagnosis of carcinoma [5]. A few studies have reported that CNL or NLR can occur in plasma cell neoplasms [67]. Here, we describe one CNL case and three NLR cases initially suspected of being CNL that were associated with solid cancers, plasma cell neoplasms or sepsis.
We retrospectively reviewed the database of bone marrow (BM) study patients from January 2014 to October 2016 and identified four patients with leukocytosis of more than 25×109/L and neutrophilia without left shift (more than 80% of segmented neutrophils). We performed a CSF3R mutation assay to help differentiate between CNL and NLR. DNA was extracted from BM aspirates, and a polymerase chain reaction (PCR) and Sanger sequencing were performed using primers for the T618I mutation on CSF3R exon 14 according to a previous study [3]: forward 5′-CCACGGAGGCAGCTTTAC-3′ and reverse 5′-AAATCAGCATCCTTTGGGTG-3′, which revealed an ACC to ATC mutation corresponding to the T618I mutation of CSF3R.
The clinical and laboratory findings of the four patients are shown in Table 1 and Fig. 1. The first patient (case 1) tested positive for CNL with CSF3R T618I. Three patients (cases 2 to 4) showed negative results for CSF3R T618I and the cause of NLR was associated with ovarian cancer, monoclonal gammopathy of undetermined significance (MGUS), and multiple myeloma (MM) with sepsis, respectively.
One patient (case 1) presented hypovolemic shock and had a history of chronic kidney disease, hypertension, and persistent leukocytosis, which had lasted for more than four months (22-61 ×109/L). Even after antibiotic treatment, his white blood cell (WBC) count had progressively increased up to 61×109/L. Three months after first admission, a BM study showed hypercellular marrow and granulocytic predominance with normal maturation (Fig. 1) along with the presence of CSF3R T618I. Taken together, he was diagnosed with CNL and was administered hydroxyurea. After 2.5 months of treatment, his WBC count normalized and remained in the normal range for a year, at the time of this study.
In case 2, diagnosed as having ovarian cancer, the patient showed uncontrolled leukocytosis and negative culture results except for Clostridium difficile. She underwent a total hysterectomy with both salpingo-oophorectomy and taxol-carboplatin chemotherapy. Although her WBC count decreased from 67×109/L to 12× 109/L immediately after surgery, it proceeded to increase up to 163.8×109/L again in one month, and an abdominal computerized tomography (CT) scan revealed newly developed multiple hepatic metastases and metastatic lymph nodes at the small bowel mesentery. Despite continuous antibiotic treatment, extreme leukocytosis (more than 100×109/L) was persistent and she expired four months after the diagnosis of ovarian cancer. Her CSF3R T618I result was found to be negative.
With solid tumors, paraneoplastic autocrine production of G-CSF can induce NLR and increase proliferation of the carcinoma. Therefore, patients with solid tumors accompanied by paraneoplastic NLR are known to incur rapid tumor growth and have poor clinical prognosis [891011]. Our patient (case 2) with NLR also showed very poor prognosis. Previous studies have demonstrated that G-CSF levels decrease after treatment of the primary tumor and WBC counts go back to nearly normal levels [12131415]. As tumors recur, NLR redevelops with an increase in G-CSF production caused by solid tumors [121314].
The other patient (case 3, MGUS with IgG-lambda type) showed neutrophilia at the time of diagnosis (Fig. 1) and throughout the ten-month follow-up period (WBC count 24-40×109/L with 83-90% segmented neutrophils) without MGUS treatment. She did not show for CSF3R T618I mutation. However, CNL can be diagnosed even in cases without any clonal markers when other diagnostic criteria are met, such as persistent neutrophilia (at least three months) and splenomegaly when no identifiable cause of NLR is present. In case 3, clinical features such as splenomegaly and persistent neutrophilia showed the likelihood of diagnosing CNL, although CSF3R T618I was negative and a cytogenetic study showed a normal karyotype. Besides CSF3R T618I mutation, CSF3R M696T or the CSF3R mutation (2341_2342insC; cytoplasmic domain) on exon 17 could be associated with CNL [316]. In addition, in CNL patients without the CSF3R T618I mutation, mutations in other genes such as ASXL1, TET2, SRSF2, JAK2, and SETBP1 have also been reported [37]. It would be helpful to examine the mutations mentioned above as well as the CSF3R T618I mutation to determine clonality, but we could not perform them.
Some studies have reported that CNL can co-occur with plasma cell neoplasm [3617] although most cases did not confirm the clonality of neutrophils [67]. It remains unclear whether neutrophilic leukocytosis is a leukemoid response caused by cytokine release such as interleukin-6 by neoplastic plasma cells or independent clonal disorders. One study has shown that about a quarter of patients with neutrophilia have coexisting MM or MGUS [7].
In case 4, the patient was transferred to our emergency room because of septic shock accompanied by extreme leukocytosis (WBC count 150×109/L with 90% segmented neutrophils). He was initially thought to have CNL rather than NLR because of his extreme leukocytosis. A BM study showed hypercellularity with severe myeloid hyperplasia (myeloid: erythroid ratio, 25.4:1). The proportion of plasma cells was only 2.4% of the total BM-nucleated cells. Plasma cell neoplasm was not suspected at first, but unexpectedly, clonal plasma cells were found by an immunophenotyping analysis. Therefore, we performed an MM workup (serum protein electrophoresis and immunofixation, MRI whole body survey, etc.). Finally, the patient was diagnosed as having MM (IgA-lambda type). He received antibiotics. Two weeks later, his WBC count was normalized to 7.4×109/L before MM treatment. Finally, the neutrophilia in this patient was thought to be caused by NLR associated with sepsis, rather than paraneoplastic syndrome, because the neutrophilia disappeared after antibiotic treatment.
In conclusion, making a diagnosis of CNL should be approached with caution in patients with coexisting solid cancer or plasma cell neoplasm, unless there is demonstrated clonality of myeloid cells, such as CSF3R T618I, other mutations, or chromosomal abnormalities. Furthermore, when infection is unlikely to be the only contributor in patients having NLR, the possibility of paraneoplastic syndromes caused by solid tumors or plasma cell neoplasms should be considered.
Figures and Tables
Fig. 1
Peripheral blood and bone marrow findings of a patient with chronic neutrophilic leukemia (case 1, A–D) and a patient with co-occurrence of neutrophilic leukemoid reaction and MGUS (case 3, E–H). They showed neutrophilia (A, E) and hypercellularity with myeloid hyperplasia in BM (B–D, F–H). A few plasma cells were noted (F, G). [PB, Wright stain, ×400 (A, E); BM aspiration, Wright stain, ×400 (B, F), ×1,000 (C, G); BM biopsy, H&E stain, ×100 (D, H)].
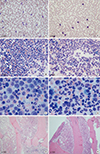
Table 1
Clinical and laboratory characteristics of patients with CNL and NLR

*nuclear-cytoplasmic asynchrony, hypogranularity, and toxic vacuole.
Abbreviations: CNL, chronic neutrophilic leukemia; CSF3R, colony stimulating factor 3 receptor; JAK2, Janus kinase 2; MGUS, monoclonal gammopathy of undetermined significance; MM, multiple myeloma; NLR, neutrophilic leukemoid reaction; NT, not tested; PDGFRA/B, Platelet-derived growth factor receptor alpha/beta.
Notes
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST No potential conflicts of interest relevant to this article were reported.
This article is available from http://www.labmedonline.org
ACKNOWLEDGMENTS
This study was supported by the Basic Science Research Program through the National Research Foundation of Korea, funded by the Ministry of Education, Science and Technology (NRF-2012-R1A1A2044138).
References
1. Granger JM, Kontoyiannis DP. Etiology and outcome of extreme leukocytosis in 758 nonhematologic cancer patients: a retrospective, single-institution study. Cancer. 2009; 115:3919–3923.

2. Halkes CJ, Dijstelbloem HM, Eelkman Rooda SJ, Kramer MH. Extreme leucocytosis: not always leukaemia. Neth J Med. 2007; 65:248–251.
3. Pardanani A, Lasho TL, Laborde RR, Elliott M, Hanson CA, Knudson RA, et al. CSF3R T618I is a highly prevalent and specific mutation in chronic neutrophilic leukemia. Leukemia. 2013; 27:1870–1873.

4. Maxson JE, Gotlib J, Pollyea DA, Fleischman AG, Agarwal A, Eide CA, et al. Oncogenic CSF3R mutations in chronic neutrophilic leukemia and atypical CML. N Engl J Med. 2013; 368:1781–1790.

5. Aliper AM, Frieden-Korovkina VP, Buzdin A, Roumiantsev SA, Zhavoronkov A. A role for G-CSF and GM-CSF in nonmyeloid cancers. Cancer Med. 2014; 3:737–746.

6. Blombery P, Kothari J, Yong K, Allen C, Gale RE, Khwaja A. Plasma cell neoplasm associated chronic neutrophilic leukemia with membrane proximal and truncating CSF3R mutations. Leuk Lymphoma. 2014; 55:1661–1662.

7. Bain BJ, Ahmad S. Chronic neutrophilic leukaemia and plasma cell-related neutrophilic leukaemoid reactions. Br J Haematol. 2015; 171:400–410.

8. Kasuga I, Makino S, Kiyokawa H, Katoh H, Ebihara Y, Ohyashiki K. Tumor-related leukocytosis is linked with poor prognosis in patients with lung carcinoma. Cancer. 2001; 92:2399–2405.

9. Kitamura H, Kodama F, Odagiri S, Nagahara N, Inoue T, Kanisawa M. Granulocytosis associated with malignant neoplasms: a clinicopathologic study and demonstration of colony-stimulating activity in tumor extracts. Hum Pathol. 1989; 20:878–885.

10. Berdel WE, Danhauser-Riedl S, Steinhauser G, Winton EF. Various human hematopoietic growth factors (interleukin-3, GM-CSF, G-CSF) stimulate clonal growth of nonhematopoietic tumor cells. Blood. 1989; 73:80–83.

11. Nakayama S, Yokote T, Iwaki K, Hirata Y, Akioka T, Miyoshi T, et al. Co-expression of multiple cytokines and their receptors in primary clear cell sarcoma of the pubic bone with features of a small round cell tumor. Int J Immunopathol Pharmacol. 2012; 25:799–804.

12. Dorn C, Bugl S, Malenke E, Muller MR, Weisel KC, Vogel U, et al. Paraneoplastic granulocyte colony-stimulating factor secretion in soft tissue sarcoma mimicking myeloproliferative neoplasia: a case report. BMC Res Notes. 2014; 7:313.

13. Kumar AK, Satyan MT, Holzbeierlein J, Mirza M, Van Veldhuizen P. Leukemoid reaction and autocrine growth of bladder cancer induced by paraneoplastic production of granulocyte colony-stimulating factor--a potential neoplastic marker: a case report and review of the literature. J Med Case Rep. 2014; 8:147.
14. Qing L, Xiang T, Guofu Z, Weiwei F. Leukemoid reaction in cervical cancer: a case report and review of the literature. BMC Cancer. 2014; 14:670.

15. Nimieri HS, Makoni SN, Madziwa FH, Nemiary DS. Leukemoid reaction response to chemotherapy and radiotherapy in a patient with cervical carcinoma. Ann Hematol. 2003; 82:316–317.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download