Abstract
Background
Workers in the microbiology laboratories are continuously exposed to the risk of laboratory-associated infections. Tuberculosis (TB) is a frequent laboratory-acquired infection owing to production of cough-generated aerosols with ease and high infectivity of Mycobacterium tuberculosis. This study aims to investigate the current situation of biosafety in Korean TB laboratories.
Methods
We conducted a nationwide survey of laboratories in hospitals conducting TB tests using questionnaires about their facility and management standards.
Results
We analyzed data from 52 hospitals nationwide that have a capacity of 100–2,000 beds, of which only two laboratories conduct high risk drug-susceptibility testing on cultured isolates among other test items, whereas six laboratories perform only direct sputum-smear microscopy. The remaining laboratories performed moderate-risk activities/tests, like sample processing for culture. In the majority of these laboratories, there are laboratory medicine specialists who are fully in charge of health checkup programs for laboratorians. The facility and management standards vary widely according to the size of the hospital and risk of TB tests.
Figures and Tables
Table 1
Characteristics of TB laboratories in medical institutions (N=52)
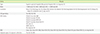
Abbreviations: TB, tuberculosis; N, number; LM specialist, laboratory medicine specialists who are in full charge of the microbiological laboratory; HCU items, test items of health checkup programs for laboratorians; TST, tuberculin skin test; IGRA, interferon-gamma release assay; BSC, biosafety cabinet.
Table 2
The distribution of tuberculosis laboratories by level of risk

*Modified from World Health Organization criteria [7].
Abbreviations: TB, tuberculosis; DST, drug-susceptibility testing.
Table 3
Compliance of facility standards in Korean tuberculosis laboratories

Table 4
Compliance of management standards in Korean tuberculosis laboratories

References
2. Acuña-Villaorduña C, White LF, Fennelly KP, Jones-López EC. Tuberculosis transmission: sputum vs aerosols. Lancet Infect Dis. 2016; 16:770–771.
3. Chiang CY, Centis R, Migliori GB. Drug-resistant tuberculosis: past, present, future. Respirology. 2010; 15:413–432.

4. World Health Organization. Laboratory Biosafety Manual, 3rd Edition 2004 [Online]. last visited on 27 July 2016. http://www.who.int/csr/delibepidemics/WHO_CDS_CSR_LYO_2004_11/en/.
5. Korea CDC. Development of biosafety guideline of clinical laboratory [Online]. last visited on 27 July 2016. http://cdc.go.kr/CDC/info/CdcKrInfo0201.jsp?menuIds=HOME001-MNU1155-MNU108&fid=28&q_type=&q_value=&cid=25661&page-Num=1.
6. Lee WG, Kwak YS, Lee DH, Hwang YS, Lee KN. Clinical pathology laboratory inspection and accreditation in Korea I: Development of the system and its trial. Korean J Clin Pathol. 2001; 21:86–92.
7. World Health Organization. Tuberculosis laboratory biosafety manual 2012 [Online]. last visited on 27 July 2016. http://apps.who.int/iris/bitstream/10665/77949/1/9789241504638_eng.pdf.
8. Cousins DV, Bastida R, Cataldi A, Quse V, Redrobe S, Dow S, et al. Tuberculosis in seals caused by a novel member of the Mycobacterium tuberculosis complex: Mycobacterium pinnipedii sp. Int J Syst Evol Microbiol. 2003; 53:1305–1314.

World Health Organization. Tuberculosis Fact sheet N°104 [Online]. last visited on 27 July 2016. http://www. who.int/mediacentre/factsheets/fs104/en/.
10. Center for Disease Control and National Institute of Health. U.S. Biosafety in Microbiological and Biomedical Laboratories. Dept of Health and Human Services. 4th Edition. Washington, DC: Government Printing Office;1999. N° 93-8395. U.S.
11. Kim SJ, Lee SH, Kim IS, Kim HJ, Kim SK, Fieder HL. Risk of occupational tuberculosis in National Tuberculosis Programme laboratories in Korea. Int J Tuberc Lung Dis. 2007; 11:138–142.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download