Abstract
Background
Pyridoxal-5′-phosphate (P5P), a coenzyme of the aspartate aminotransferase (AST) and alanine aminotransferase (ALT) reactions, is required to measure aminotransferase levels (IFCC method). However, a modified IFCC method that uses a reagent devoid of P5P is commonly used in laboratories in Korea. To determine the differences between the two methods, we compared aminotransferase levels measured by using the IFCC method and modified IFCC method.
Methods
Serum levels of AST and ALT, with and without P5P, were measured in 2,318 patients. Based on the allowable limits of performance set by the Royal College of Pathologists of Australasia (RCPA), differences between the two methods were analyzed under various conditions.
Results
Higher AST and ALT values were obtained by the IFCC method compared to modified IFCC method, showing significant differences between the two methods (AST, 5.8±14.2 IU/L; ALT, 2.8±6.9 IU/L) (P<0.001). Values exceeding RCPA criteria were more frequently observed in emergency orders (AST, 65.8%; ALT, 14.4%) than in routine orders (AST, 3.2%; ALT, 9.6%), as well as in inpatient wards (AST, 70.4%; ALT, 18.5%) compared to outpatient clinics (AST, 56.6%; ALT, 10.0%). However, the differences between the two methods were not significant among the disease groups, except for the acute myocardial infarction group.
Conclusions
The method using reagents without P5P underestimated aminotransferase activity. The effect of P5P was more significant in patients with acute myocardial infarction, considered as P5P-deficient. In conclusion, the IFCC method with P5P should be applied for measuring AST and ALT serum levels.
Go to : 
REFERENCES
1. McPherson RA, Pincus MR. Henry's clinical diagnosis and management by laboratory methods. Elsevier Health Sciences. 2016. 295–6.
2. Schumann G, Bonora R, Ceriotti F, Ferard G, Ferrero CA, Franck PF, et al. IFCC primary reference procedures for the measurement of catalytic activity concentrations of enzymes at 37 degrees C. International Federation of Clinical Chemistry and Laboratory Medicine. Part 4. Reference procedure for the measurement of catalytic concentration of alanine aminotransferase. Clin Chem Lab Med. 2002; 40:718–24.

3. Schumann G, Bonora R, Ceriotti F, Ferard G, Ferrero CA, Franck PF, et al. IFCC primary reference procedures for the measurement of catalytic activity concentrations of enzymes at 37 degrees C. International Federation of Clinical Chemistry and Laboratory Medicine. Part 5. Reference procedure for the measurement of catalytic concentration of aspartate aminotransferase. Clin Chem Lab Med. 2002; 40:725–33.

4. Horder M, Moore RE, Bowers GN Jr. Aspartate aminotransferase activity in human serum. Factors to be considered in supplementation with pyridoxal 5'-phosphate in vitro. Clin Chem. 1976; 22:1876–83.

5. Jun SH, Song J. Annual report on the external quality assessment scheme for clinical chemistry in Korea (2014). J Lab Med Qual Assur. 2015; 37:115–23.

6. Chasan-Taber L, Selhub J, Rosenberg IH, Malinow MR, Terry P, Tishler PV, et al. A prospective study of folate and vitamin B6 and risk of myocardial infarction in US physicians. J Am Coll Nutr. 1996; 15:136–43.

7. Roubenoff R, Roubenoff RA, Selhub J, Nadeau MR, Cannon JG, Freeman LM, et al. Abnormal vitamin B6 status in rheumatoid cachexia. Association with spontaneous tumor necrosis factor alpha production and markers of infammation. Arthritis Rheum. 1995; 38:105–9.
8. Kok FJ, Schrijver J, Hofman A, Witteman JC, Kruyssen DA, Remme WJ, et al. Low vitamin B6 status in patients with acute myocardial infarction. Am J Cardiol. 1989; 63:513–6.
9. Jones GR, Sikaris K, Gill J. ‘Allowable limits of performance’ for external quality assurance programs - an approach to application of the Stockholm Criteria by the RCPA Quality Assurance Programs. Clin Biochem Rev. 2012; 33:133–9.
10. Tutor-Crespo MJ, Hermida J, Tutor JC. Activation of serum aminotransferases by pyridoxal-5´-phosphate in epileptic patients treated with anticonvulsant drugs. Clin Biochem. 2004; 37:714–7.

11. Gressner AM, Sittel D. Plasma pyridoxal 5´-phosphate concentrations in relation to apo-aminotransferase levels in normal, uraemic, and post-myocardial infarct sera. J Clin Chem Clin Biochem. 1985; 23:631–6.

12. Ross AC. Modern nutrition in health and disease. Wolters Kluwer Health/Lippincott Williams & Wilkins. 2014. 452–9.
13. Institute of Medicine Standing Committee on the Scientifc Evaluation of Dietary Reference I, its Panel on Folate OBV, Choline. The National Academies Collection: Reports funded by National Institutes of Health. Dietary Reference Intakes for Thiamin, Ribofavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. Washington (DC): National Academies Press (US) National Academy of Sciences;1998.
14. Molina-Lopez J, Florea D, Quintero-Osso B, de la Cruz AP, Rodriguez-Elvira M, Del Pozo EP. Pyridoxal-5'-phosphate defciency is associated with hyperhomocysteinemia regardless of antioxidant, thiamine, ribofavin, cobalamine, and folate status in critically ill patients. Clin Nutr. 2016; 35:706–12.
15. Jamieson CP, Obeid OA, Powell-Tuck J. The thiamin, ribofavin and pyridoxine status of patients on emergency admission to hospital. Clin Nutr. 1999; 18:87–91.
16. Labadarios D, Brink PA, Weich HF, Visser L, Louw ME, Shephard GS, et al. Plasma vitamin A, E, C and B6 levels in myocardial infarction. S Af Med J. 1987; 71:561–3.
17. Giannini EG, Testa R, Savarino V. Liver enzyme alteration: a guide for clinicians. CMAJ. 2005; 172:367–79.

18. CLIA Requirements for Analytical Quality. https://www.westgard.com/clia.htm.
19. Ricos C, Alvarez V, Cava F, Garcia-Lario JV, Hernandez A, Jimenez CV, et al. Desirable Specifcations for Total Error, Imprecision, and Bias, derived from intra- and inter-individual biologic variation. https://www.westgard.com/biodatabase1.htm. (Updated on 2014).
Go to : 
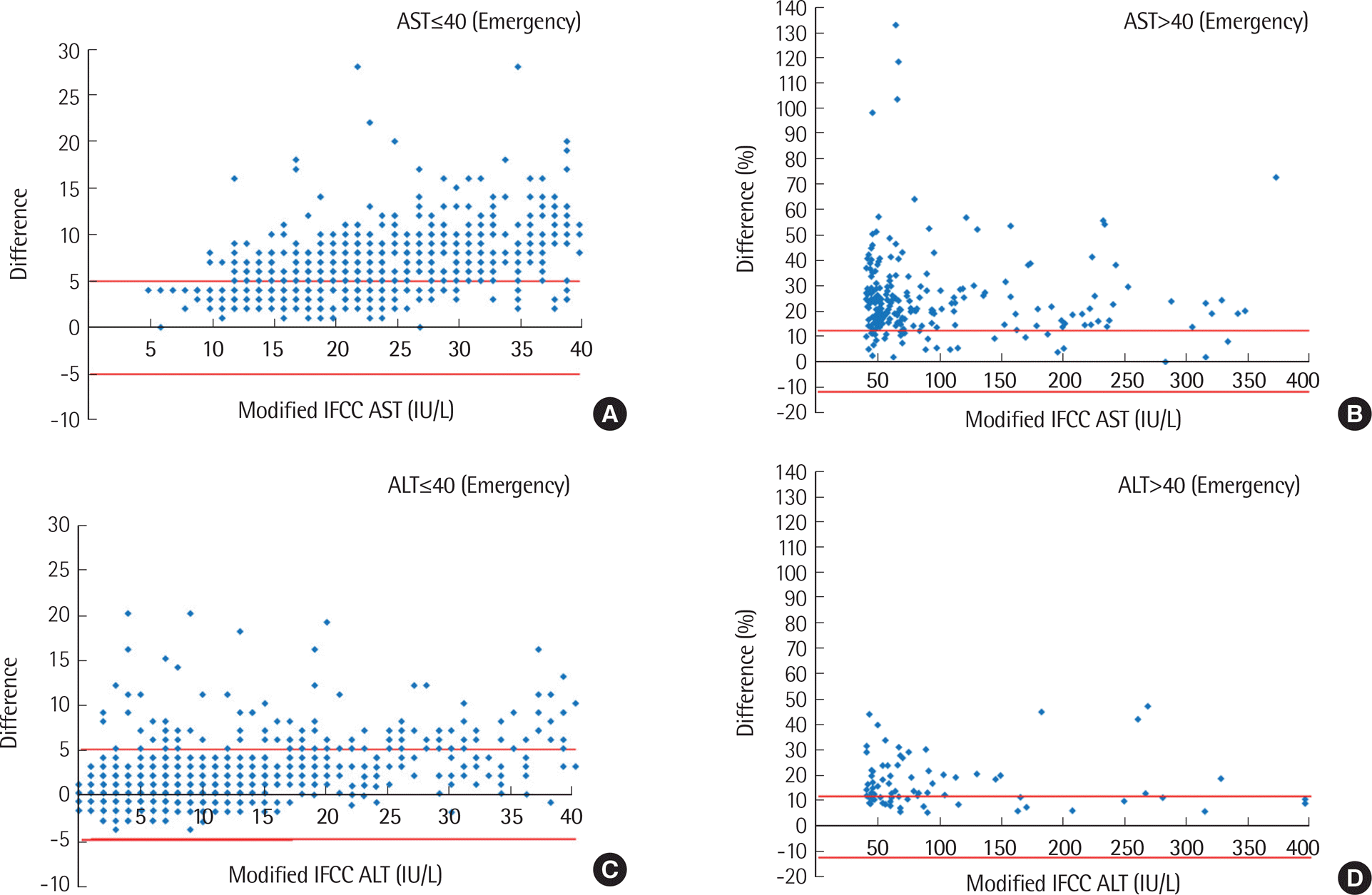 | Fig. 1.Bland-Altman plots for AST (IU/L) (A and B) and ALT (IU/L) (C and D) performed as emergency tests. Red lines represent allowable limits of performance. |
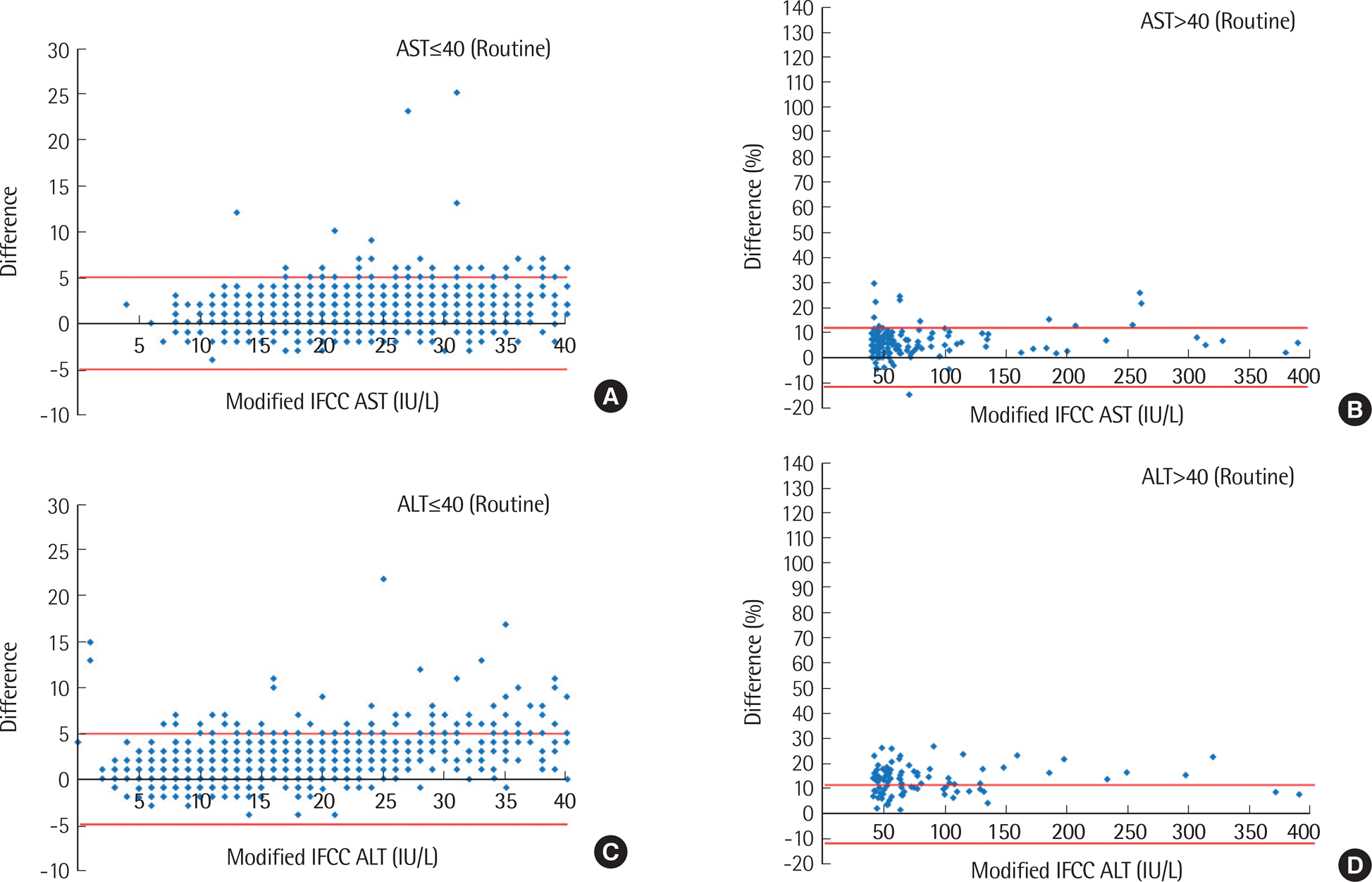 | Fig. 2.Bland-Altman plots for AST (IU/L) (A and B) and ALT (IU/L) (C and D) performed as routine tests. Red lines represent allowable limits of performance. |
Table 1.
Aminotransferase levels measured by the two methods
| IFCC | Modified IFCC | Difference∗ (IU/L) | P-value | |
|---|---|---|---|---|
| AST (IU/L) | 41.6±62.1 | 35.7±51.3 | 5.8±14.2 | <0.001 |
| ALT (IU/L) | 22.4±35.8 | 19.6±30.6 | 2.8±6.9 | <0.001 |
Table 2.
Distributions of aminotransferase results in subgroups according to the types of order and status
| Group | AST | ALT | ||||
|---|---|---|---|---|---|---|
| Difference∗ (IU/L) | Exceed the limits % | P value | Difference∗ (IU/L) | Exceed the limits N (%) | P value | |
| Type of order | <0.001 | <0.001 | ||||
| Emergency order | 11.1±19.9 | 65.8 (648/985) | 3.0±8.1 | 14.4 (142/985) | ||
| Routine order | 1.9±5.0 | 3.2 (43/1333) | 2.6±5.8 | 9.6 (128/1333) | ||
| Status | 0.001 | 0.004 | ||||
| Inpatient ward | 13.4±20.9 | 70.4 (190/270) | 4.1±12.5 | 18.5 (50/270) | ||
| Emergency room | 13.6±38.1 | 65.2 (86/132) | 2.0±3.7 | 9.8 (13/132) | ||
| Outpatient clinic | 7.6±7.8 | 56.6 (141/249) | .0±3.2 | 10.0 (25/249) | ||
Table 3.
Distribution ns of aminotransferase r esults in subgroups accordi ing to various diseases
| Disease | AST | ALT | ||||
|---|---|---|---|---|---|---|
| Difference∗ (IU/L) | Exceed the limits, % | P value | Difference∗ (IU/L) | Exceed the limits, % | P value | |
| CKD | 0.121 | 0.059 | ||||
| Yes | 6.8±4.1 | 47.8 (11/23) | 0.5±2.0 | 0.0 (0/23) | ||
| No | 11.4±22.9 | 64.6 (406/628) | 2.9±8.6 | 14.0 (88/628) | ||
| Liver disease | 0.295 | 0.054 | ||||
| Yes | 20.8±39.4 | 71.2 (37/52) | 9.1±25.1 | 23.1 (12/52) | ||
| No | 10.4±20.2 | 63.4 (380/599) | 2.3±4.6 | 12.7 (76/599) | ||
| Malignancy | 0.141 | 0.253 | ||||
| Yes | 10.1±18.6 | 61.2 (200/327) | 3.0±10.7 | 11.9 (39/327) | ||
| No | 12.4±25.8 | 67.0 (217/324) | 2.7±5.4 | 15.1 (49/324) | ||
| Infection | 0.115 | 0.461 | ||||
| Yes | 10.9±13.1 | 54.9 (39/71) | 3.0±5.9 | 9.9 (7/71) | ||
| No | 11.3±23.4 | 65.2 (378/580) | 2.8±8.8 | 14.0 (81/580) | ||
| AMI | <0.001 | 0.002 | ||||
| Yes | 41.0±82.0 | 100.0 (23/23) | 6.0±5.9 | 39.1 (9/23) | ||
| No | 10.1±16.0 | 62.7 (394/628) | 2.7±8.6 | 12.6 (79/628) | ||
| Other diseases | 0.161 | 0.140 | ||||
| Yes | 10.4±13.0 | 68.0 (140/206) | 2.7±5.6 | 16.5 (34/206) | ||
| No | 11.6±25.7 | 62.2 (277/445) | 2.9±9.5 | 12.1 (54/445) | ||
Table 4.
Frequencies of exceeded limits according to three criteria




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download