Abstract
Background
We evaluated three commercially available vitamin D assays to evaluate and compare the correlation and accuracy among them.
Methods
Vitamin D was measured in 71 patient samples using the Architect 25-OH vitamin D assay (Abbott), the ADVIA Centaur vitamin D total assay (Siemens), and the LIAISON 25 OH vitamin D total assay (Diasorin). The evaluation made use of both patient samples and standard reference material, SRM 972. To analyze correlations and differences, Pearson's correlation coefficients and paired sample t-tests were performed.
Results
Correlations among the three evaluated assays showed strong positive linear relationships (correlations among Siemens and DiaSorin, DiaSorin and Abbott, Abbott and Siemens: r=0.935, r=0.927, r=0.909, respectively). Mean (SD) vitamin D values on Siemens, Abbott, and DiaSorin assays in the 71 patient samples were 23.09 (10.41), 16.75 (11.26), and 16.76 (9.32), respectively. Results for the Siemens assay were significantly different from the other two methods (P<0.001). Target values for SRM 972 level 1, 2, 3, and 4 were 23.9, 14.0, 44.9, and 35.4, respectively. The Abbott, Siemens, and Diasorin assay values were closest to the target values in level 1, levels 2 and 3, and level 4, respectively.
Conclusions
Correlations among vitamin D assays were good; however, the mean values of the Siemens assay were significantly higher than those of DiaSorin or Abbott. We found significant differences in vitamin D levels and discrepancies between patient samples and SRM 972 samples, which should be considered during use in a clinical setting.
Go to : 
REFERENCES
1. Audran M, Kumar R. The physiology and pathophysiology of vitamin D. Mayo Clin Proc. 1985; 60:851–66.

2. Chlebowski RT. Vitamin D and breast cancer: interpreting current evidence. Breast Cancer Res. 2011; 13:217.

3. Chiang KC, Yeh CN, Chen MF, Chen TC. Hepatocellular carcinoma and vitamin D: a review. J Gastroenterol Hepatol. 2011; 26:1597–603.

4. Woloszynska-Read A, Johnson CS, Trump DL. Vitamin D and cancer: clinical aspects. Best Pract Res Clin Endocrinol Metab. 2011; 25:605–15.

5. Choi HS, Kim KA, Lim CY, Rhee SY, Hwang YC, Kim KM, et al. Low serum vitamin D is associated with high risk of diabetes in Korean adults. J Nutr. 2011; 141:1524–8.

6. Holick MF. Vitamin D defciency. N Engl J Med. 2007; 357:266–81.
7. Nair R, Maseeh A. Vitamin D: The “sunshine” vitamin. J Pharmacol Pharmacother. 2012; 3:118–26.
8. DeLuca HF. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr. 2004; 80:1689S–96S.

9. Dawson-Hughes B, Heaney RP, Holick MF, Lips P, Meunier PJ, Vieth R. Estimates of optimal vitamin D status. Osteoporos Int. 2005; 16:713–6.

10. Holick MF. Vitamin D status: measurement, interpretation, and clinical application. Ann Epidemiol. 2009; 19:73–8.

11. Hsu JJ, Tintut Y, Demer LL. Vitamin D and osteogenic differentiation in the artery wall. Clin J Am Soc Nephrol. 2008; 3:1542–7.

12. Medici D, Razzaque MS, Deluca S, Rector TL, Hou B, Kang K, et al. FGF-23-Klotho signaling stimulates proliferation and prevents vitamin D-induced apoptosis. J Cell Biol. 2008; 182:459–65.

13. Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvi-tamin D for multiple health outcomes. Am J Clin Nutr. 2006; 84:18–28.

14. Carter GD, Carter R, Jones J, Berry J. How accurate are assays for 25-hy-droxyvitamin D? Data from the international vitamin D external quality assessment scheme. Clin Chem. 2004; 50:2195–7.

15. Farrell C, Soldo J, Williams P, Herrmann M. 25-Hydroxyvitamin D testing: challenging the performance of current automated immunoassays. Clin Chem Lab Med. 2012; 50:1953–63.

16. Heaney RP, Recker RR, Grote J, Horst RL, Armas LA. Vitamin D(3) is more potent than vitamin D(2) in humans. J Clin Endocrinol Metab. 2011; 96:E447–52.

17. Holick MF, Biancuzzo RM, Chen TC, Klein EK, Young A, Bibuld D, et al. Vitamin D2 is as effective as vitamin D3 in maintaining circulating concentrations of 25-hydroxyvitamin D. J Clin Endocrinol Metab. 2008; 93:677–81.

18. Yu S, Cheng X, Fang H, Zhang R, Han J, Qin X, et al. 25OHD analogues and vacuum blood collection tubes dramatically affect the accuracy of automated immunoassays. Sci Rep. 2015; 5:14636.

19. Heijboer AC, Blankenstein MA, Kema IP, Buijs MM. Accuracy of 6 routine 25-hydroxyvitamin D assays: infuence of vitamin D binding protein concentration. Clin Chem. 2012; 58:543–8.
20. Lee JH, Choi JH, Kweon OJ, Park AJ. Discrepancy between vitamin D total immunoassays due to various cross-reactivities. J Bone Metab. 2015; 22:107–12.

21. Enko D, Fridrich L, Rezanka E, Stolba R, Ernst J, Wendler I, et al. 25-hydroxy-Vitamin D status: limitations in comparison and clinical interpretation of serum-levels across different assay methods. Clin Lab. 2014; 60:1541–50.

22. Wallace AM, Gibson S, de la Hunty A, Lamberg-Allardt C, Ashwell M. Measurement of 25-hydroxyvitamin D in the clinical laboratory: current procedures, performance characteristics and limitations. Steroids. 2010; 75:477–88.

23. Abbott ARCHITECT® 25-OH Vitamin D Assay [directional insert 3L52]. Abbott Laboratories AP, IL, USA (2010). Abbott Labratories; Abbott Park, IL, USA. 2010.
24. Siemens ADVIA Centaur® ACXVDTVAdiERA, 2013-07] Siemens Healthcare Diagnostics Inc., Tarrytown, NY, USA. 2013.
25. Freeman J, Wilson K, Spears R, Shalhoub V, Sibley P. Infuence of vitamin D binding protein on accuracy of 25-hydroxyvitamin D measurement using the ADVIA Centaur Vitamin D Total Assay. Int J Endocrinol. 2014; 2014:691679.
26. Sinotte M, Diorio C, Berube S, Pollak M, Brisson J. Genetic polymorphisms of the vitamin D binding protein and plasma concentrations of 25-hydroxyvitamin D in premenopausal women. Am J Clin Nutr. 2009; 89:634–40.

27. Kamboh MI, Ferrell RE. Ethnic variation in vitamin D-binding protein (GC): a review of isoelectric focusing studies in human populations. Hum Genet. 1986; 72:281–93.

28. Santos BR, Mascarenhas LP, Boguszewski MC, Spritzer PM. Variations in the vitamin D-binding protein (DBP) gene are related to lower 25-hy-droxyvitamin D levels in healthy girls: a cross-sectional study. Horm Res Paediatr. 2013; 79:162–8.

29. Park Y, Kim YS, Kang YA, Shin JH, Oh YM, Seo JB, et al. Relationship between vitamin D-binding protein polymorphisms and blood vitamin D level in Korean patients with COPD. Int J Chron Obstruct Pul-mon Dis. 2016; 11:731–8.
30. Moon HW, Cho JH, Hur M, Song J, Oh GY, Park CM, et al. Comparison of four current 25-hydroxyvitamin D assays. Clin Biochem. 2012; 45:326–30.

31. Wyness SP, Straseski JA. Performance characteristics of six automated 25-hydroxyvitamin D assays: Mind your 3s and 2s. Clin Biochem. 2015; 48:1089–96.

32. Li L, Zeng Q, Yuan J, Xie Z. Performance evaluation of two immunoassays for 25-hydroxyvitamin D. J Clin Biochem Nutr. 2016; 58:186–92.

33. Hsu SA, Soldo J, Gupta M. Evaluation of two automated immunoassays for 25-OH vitamin D: comparison against LC-MS/MS. J Steroid Biochem Mol Biol. 2013; 136:139–45.

34. Chen Y, Kinney L, Bozovic A, Smith H, Tarr H, Diamandis EP, et al. Performance evaluation of Siemens ADVIA Centaur and Roche MOD-ULAR Analytics E170 Total 25-OH Vitamin D assays. Clin Biochem. 2012; 45:1485–90.

35. Saenger AK, Laha TJ, Bremner DE, Sadrzadeh SM. Quantifcation of serum 25-hydroxyvitamin D(2) and D(3) using HPLC-tandem mass spectrometry and examination of reference intervals for diagnosis of vitamin D defciency. Am J Clin Pathol. 2006; 125:914–20.
Go to : 
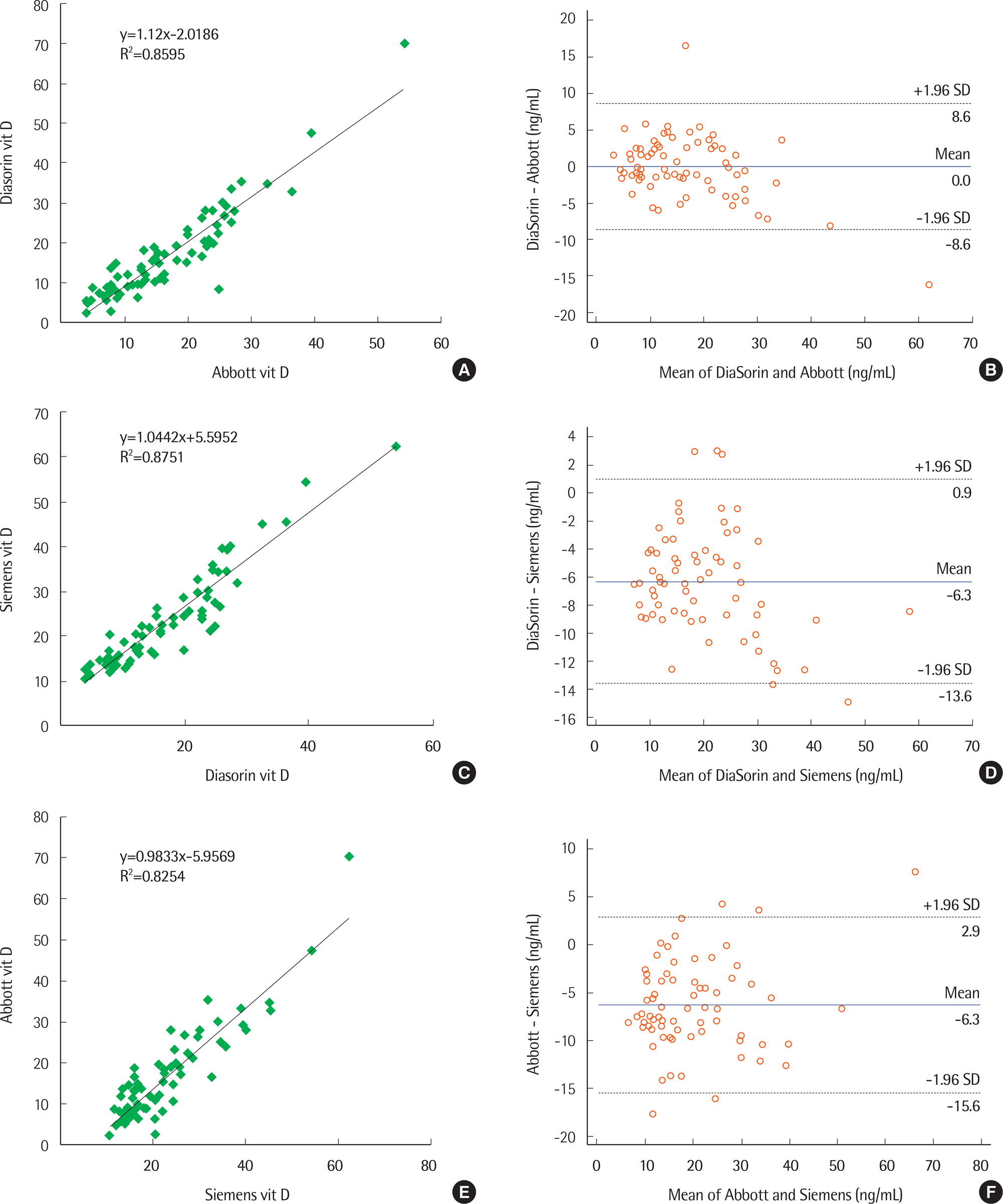 | Fig. 1.Linear regression and Bland-Altman analyses of immunoassays. (A) and (B), Abbott and DiaSorin; (C) and (D), DiaSorin and Siemens; and (E) and (F), Abbott and Siemens. Abbreviations: Abbott, Architect 25-OH vitamin D assay of Abbott; DiaSorin, LIAISON 25 OH vitamin D total assay of DiaSorin; Siemens, ADVIA Centaur vitamin D total assay of Siemens. |
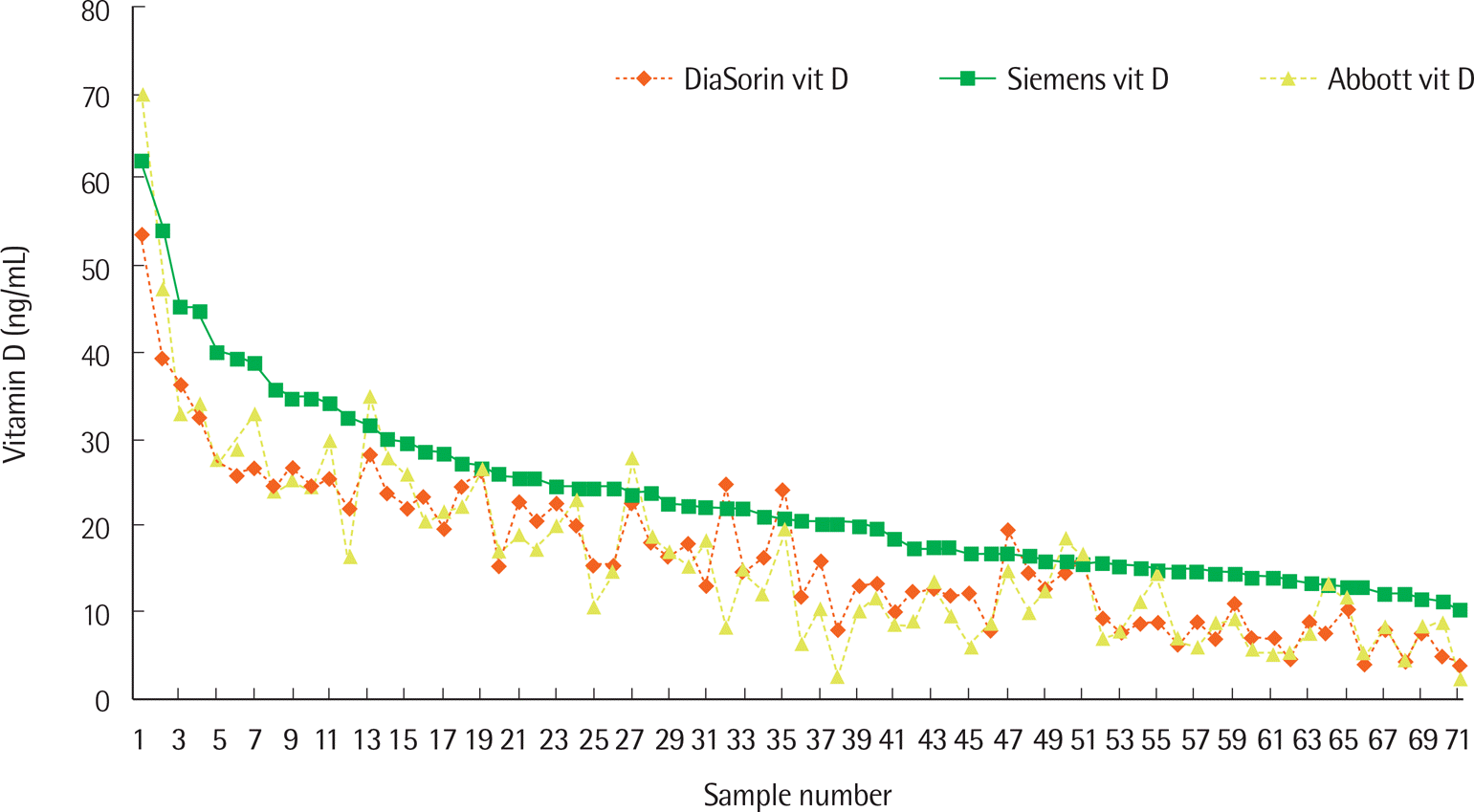 | Fig. 2.Distribution of vitamin D levels of all samples by Siemens, Abbott, and DiaSorin. The mean (SD) of Siemens, Abbott, and DiaSorin assays was 23.09 (10.41), 16.75 (11.26), and 16.76 (9.32), respectively. Siemens (■), Abbott (▲), and DiaSorin (♦) indicate the ADVIA Centaur vitamin D total assay of Siemens, the Architect 25-OH vitamin D assay of Abbott, and the LIAISON 25 OH vitamin D total assay of DiaSorin, respectively. |
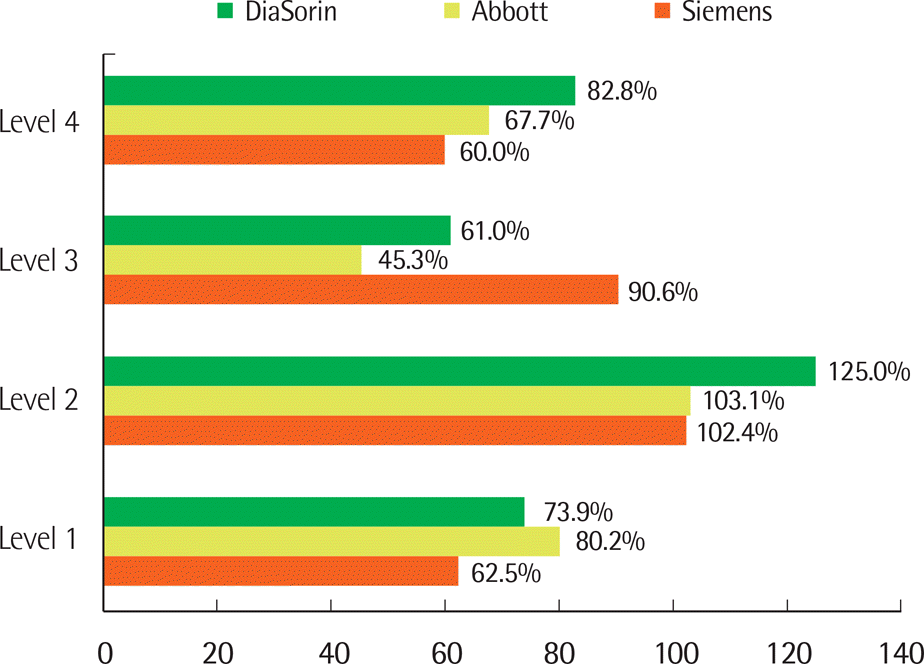 | Fig. 3.Measured values relative to target values for vitamin D. Target values for SRM 972 levels 1, 2, 3, and 4 were 23.9, 14.0, 44.9, and 35.4, respectively. Siemens (■), Abbott (■), and DiaSorin (■) indicate the ADVIA Centaur vitamin D total assay of Siemens, the Architect 25-OH vitamin D assay of Abbott, and the LIAISON 25 OH vitamin D total assay of DiaSorin, respectively. |
Table 1.
Paired sample t-tests among Siemens, Abbott, and DiaSorin vitamin D assays
The difference between the paired assays is smaller between DiaSorin and Abbott compared to that between Siemens and each of the other two assays, and the DiaSorin – Abbott difference is not significant. In contrast, the differences between DiaSorin and Siemens and between Abbott and Siemens assays were significant.
Table 2.
Accuracy of the DiaSorin, Abbott, and Siemens vitamin D assays




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download