Abstract
Background
Methods
Results
REFERENCES
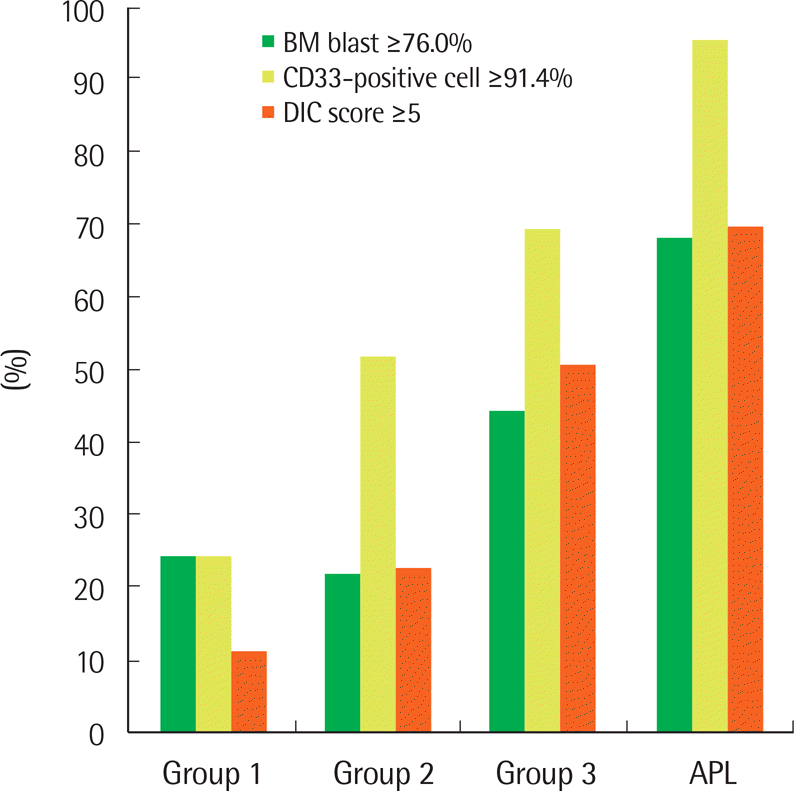 | Fig. 1.The distribution and frequency of cases with high bone marrow blasts, CD33-positive cells, and DIC score. Patients with APL-like AML (group 3) showed an intermediate feature between HLA-DR+/CD34+ (group 1) and true APL cases. The cutoff values for bone marrow blasts (76.0%) and CD33-positive cells (91.4%) were derived from values corresponding to the 75 percentile of patients with group 1. The cutoff value for DIC score (5) was based on the ISTH consensus proposal. The frequencies of BM blast ≥76.0% were 25.2% in group 1, 22.6% in group 2, 45.0% in group 3, and 69.1% in APL. The frequencies of CD33-positive cell ≥91.4% were 25.2% in group 1, 52.6% in group 2, 70.0% in group 3, and 96.3% in APL. The frequencies of ISTH DIC score ≥5 were 12.3% in group 1, 23.6% in group 2, 51.5% in group 3, and 70.4% in APL. All of these three variables (BM blast, CD33-positive cell, DIC score) were significant (each, P<0.001) by Chi-square test for trends. |
Table 1.
| Group 1 (N=294) | Group 2 (N=133)† | Group 3 (N=40)‡ | APL (N=55) | P value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 v 2 | 1 v 3 | 2 v 3 | 1 v APL | 2 v APL | 3 v APL | |||||
| Age, yr (range) | 56.5 (3-83) | 57 (0.1–90) | 59 (1–80) | 46 (7–74) | 0.317 | 0.767 | 0.808 | <0.0001 | 0.002 | 0.012 |
| Pediatric age (%) | 16 (5.4) | 11 (8.3) | 3 (7.5) | 2 (3.6) | 0.369 | 0.870 | 0.862 | 0.823 | 0.410 | 0.713 |
| Male sex (%) | 184 (62.6) | 72 (54.1) | 23 (57.5) | 22 (40.0) | 0.123 | 0.654 | 0.846 | 0.003 | 0.109 | 0.139 |
| WBC count, ×109/L (range) | 4.45 (0.5–455) | 13.2 (0.3–363.4) | 17.8 (1.4–144.3) | 5.1 (0.4–196.4) | 0.0001 | 0.0001 | 0.192 | 0.842 | 0.032 | 0.005 |
| Hemoglobin level, g/dL (range) | 8.8 (3.3–17.3) | 8.5 (3.7–13.0) | 8.75 (4.1–13.5) | 8.5 (4.6–11.9) | 0.479 | 0.835 | 0.595 | 0.188 | 0.429 | 0.327 |
| Platelet count, ×109/L (range) | 55.5 (3–1019) | 75 (4–796) | 58.5 (11–312) | 40 (7–135) | 0.028 | 0.477 | 0.685 | 0.023 | 0.0003 | 0.035 |
| BM blast, % (range) | 49.6 (6.6–96.2) | 53.2 (3.0–93.2) | 73.1 (16.0–94.0) | 83.6 (25.6–96.6) | 0.573 | 0.0003 | 0.0007 | <0.0001 | <0.0001 | 0.012 |
| FAB subtype (%) | <0.0001 | 0.174 | 0.008 | |||||||
| M0 | 19 (6.5) | 3 (2.3) | 2 (5.0) | |||||||
| M1 | 89 (30.3) | 29 (21.8) | 20 (50.0) | |||||||
| M2 | 146 (49.7) | 43 (32.3) | 12 (30.0) | |||||||
| M4 | 17 (5.8) | 32 (24.1) | 3 (7.5) | |||||||
| M5 | 3 (1.0) | 14 (10.5) | 1 (2.5) | |||||||
| M6 | 13 (4.4) | 7 (5.3) | 2 (5.0) | |||||||
| M7 | 7 (2.4) | 5 (3.8) | 0 | |||||||
| WHO subtype (%) | 0.0003 | 0.009 | <0.0001 | |||||||
| AML, NOS | 145 (49.3) | 48 (36.1) | 30 (75.0) | |||||||
| AML with MRC | 127 (43.2) | 83 (62.4) | 8 (20.0) | |||||||
| tAML | 22 (7.5) | 2 (1.5) | 2 (5.0) | |||||||
| Cytogenetics (%)§ | ||||||||||
| NK | 102/289 (35.3) | 63/130 (48.5) | 26/37 (70.3) | 0.015 | 0.0001 | 0.031 | ||||
| Complex | 71/289 (24.6) | 17/130 (13.1) | 4/37 (10.8) | 0.011 | 0.094 | 0.932 | ||||
| MK | 63/289 (21.8) | 14/130 (10.8) | 3/37 (8.1) | 0.011 | 0.084 | 0.870 | ||||
| Cytogenetic risk | 0.0006 | 0.008 | 0.517 | |||||||
| Poor | 98/289 (33.9) | 22/130 (16.9) | 4/37 (10.8) | |||||||
| Intermediate | 191/289 (66.1) | 108/130 (83.1) | 33/37 (89.2) | |||||||
| Mutations (%) | ||||||||||
| FLT3-ITD | 44/291 (15.1) | 29/132 (22.0) | 9/39 (23.1) | 12 (21.8) | 0.112 | 0.299 | 0.942 | 0.300 | 0.864 | 0.915 |
| NPM1 | 13/274 (4.7) | 42/126 (33.3) | 28/37 (75.7) | <0.0001 | <0.0001 | <0.0001 | ||||
| CEBPAll | 17/179 (9.5) | 0/80 | 0/21 | 0.010 | 0.288 | NA | ||||
| MLL-PTD | 8/124 (6.5) | 6/66 (9.1) | 0/11 | 0.710 | 0.840 | 0.664 | ||||
§ We excluded eleven cases with suboptimal metaphase (<20 metaphase with no cytogenetic abnormalities) from the analysis;
ll Only biallelic CEBPA mutations were calculated. Abbreviations: APL, acute promyelocytic leukemia; v, versus; WBC, white blood cell; BM, bone marrow; FAB, French-American-British; NOS, not otherwise specified; MRC, myelodysplasia-related changes; tAML, therapy-related acute myeloid leukemia; NK, normal karyotype; MK, monosomal karyotype; ITD, internal tandem duplication; NA, not available; PTD, partial tandem duplication.
Table 2.
| Group 1∗ (N=294) | Group 2 (N=133)† | Group 3 (N=40)‡ | APL (N=55) | P value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 v 2 | 1 v 3 | 2 v 3 | 1 v APL | 2 v APL | 3 v APL | |||||
| CD117, N (%) | 283 (96.3) | 79 (59.4) | 31 (77.5) | 53 (96.4) | <0.0001 | <0.0001 | 0.058 | 0.726 | 0.0001 | 0.012 |
| CD33, N (%) | 277 (94.2) | 130 (97.7) | 39 (97.5) | 55 (100.0) | 0.177 | 0.625 | 0.610 | 0.137 | 0.629 | 0.872 |
| CD13, N (%) | 284 (96.6) | 120 (90.2) | 37 (92.5) | 55 (100.0) | 0.014 | 0.411 | 0.901 | 0.343 | 0.037 | 0.142 |
| CD33, % (range)§ | 76.65 (0.8–99.5) | 91.8 (5.3–99.5) 9 | 96.05 (23.2–99.6) | 98.6 (48.7–99.9) | <0.0001 | <0.0001 | 0.012 | <0.0001 | <0.0001 | 0.0001 |
| CD13, % (range)§ | 81.7 (1.8–99.6) | 69.7 (0.9–98.9) | 56.8 (3.6–97.9) | 93.5 (41.1–99.7) | <0.0001 | <0.0001 | 0.133 | <0.0001 | <0.0001 | <0.0001 |
| CD33%/CD13%, ratio (range) | 1.0 (0-50.7) | 1.2 (0.1–60.5) | 1.7 (0.3–26.3) | 1.0 (0.6–2.4) | <0.0001 | <0.0001 | 0.023 | <0.0001 | 0.009 | <0.0001 |
| CD65, N (%) | 118 (40.1) | 85 (63.9) | 14 (35.0) | 42 (76.4) | <0.0001 | 0.652 | 0.002 | <0.0001 | 0.137 | 0.0001 |
| CD15, N (%) | 158 (53.7) | 98 (73.7) | 18 (45.0) | 27 (49.1) | 0.0002 | 0.384 | 0.001 | 0.626 | 0.002 | 0.852 |
| CLEll | ||||||||||
| CD56, N (%) | 40 (13.6) | 23 (17.3) | 11 (27.5) | 8 (14.5) | 0.397 | 0.040 | 0.231 | 0.978 | 0.806 | 0.194 |
| CD2, N (%) | 20 (6.8) | 5 (3.8) | 1 (2.5) | 22 (40.0) | 0.309 | 0.481 | 0.912 | <0.0001 | <0.0001 | 0.0001 |
| CD7, N (%) | 73 (24.8) | 12 (9.0) | 1 (2.5) | 4 (7.3) | 0.0003 | 0.003 | 0.303 | 0.007 | 0.917 | 0.573 |
| TdT, N (%) | 15 (5.1) | 1 (0.8) | 0 | 0 | 0.055 | 0.292 | 0.523 | 0.177 | 0.648 | NA |
| Others, N (%) | 8 (2.7) | 6 (4.5) | 1 (2.5) | 0 | 0.504 | 0.660 | 0.914 | 0.455 | 0.252 | 0.872 |
§ The percentage (%) indicates the proportion of CD33 or CD13-positive cells among the total number of cells tested;
ll The distributions of cross-lineage antigen expression may overlap with each other. For example, the patient expressing both CD56 and CD7 was categorized as CD56 and CD7. Abbreviations: APL, acute promyelocytic leukemia; v, versus; N, number of a positive case; CLE, cross-lineage expression; NA, not available.
Table 3.
| Group 1∗ | Group 2† | Group 3‡ | APL | P value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (N=294) | (N=133) | (N=40) | (N=55) | 1 v 2 | 1 v 3 | 2 v 3 | 1 v APL | 2 v APL | 3 v APL | |
| Fibrinogen (mg/dL)§ | 350 (54-845) | 367 (28–907) | 308 (52–771) | 123.5 (5–384) | 0.669 | 0.009 | 0.012 | <0.0001 | <0.0001 | <0.0001 |
| FDP (μg/mL)ll | 5.1 (0–87.6) | 6.3 (0–120.0) | 33.45 (4.2–102.9) | 47.95 (5.6–130.1) | 0.094 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.011 |
| D-dimer (μg/mL FEU)¶ | 1.14 (0.06–39.50) | 1.765 (0.16–59.10) | 15.25 (0.27-321.0) | 18.95 (2.12–494.0) | 0.016 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.534 |
| DIC score ≥5, N (%)∗∗ | 23 (12.3) | 21 (23.6) | 17 (51.5) | 38 (70.4) | 0.026 | <0.0001 | 0.006 | <0.0001 | <0.0001 | 0.123 |
§ Test results were available for 190 patients of group 1, 89 of group 2, 34 of group 3, and 54 of APL;
ll Test results were available for 167 patients of group 1, 83 of group 2, 30 of group 3, and 52 of APL;




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download