Abstract
Recent advances in chemotherapy have led to increased survival rates for patients with hematologic malignancies. However, standard chemotherapies, including alkylating agents for non-Hodgkin lymphoma, could induce therapy-related myeloid neoplasms (t-MNs), a group of disorders categorized by the World Health Organization in 2008. Here, we report a case of coexistence of bone marrow (BM)-involved refractory marginal zone B-cell lymphoma (MZL) and therapy-related myelodysplastic syndrome (t-MDS). Simultaneous presence of refractory lymphoma and t-MN in the BM is rare, and this is the first report in Korea. The patient received allogeneic hematopoietic stem cell transplantation (HSCT) to cure both the MZL and t-MDS. Since the HSCT, he has been stable for 21 months without any evidence of recurrence.
Recent advances in chemotherapy and radiotherapy have led to increased survival rates for patients with cancer, including hematologic malignancies. Therapy-related myeloid neoplasms (t-MNs), as defined by the World Health Organization (WHO), encompass therapy-related myelodysplastic syndrome (t-MDS) and therapy-related acute myeloid leukemia (t-AML), and constitute the most significant complication caused by cytotoxic chemotherapy [1]. In indolent non-Hodgkin lymphoma (NHL), t-MNs have also been recognized as a fatal complication of chemotherapy, with significantly increased incidence [2]. Despite the prolonged survival of patients with indolent lymphomas due to the incorporation of immunochemotherapeutic drugs such as rituximab [3], repeated chemotherapies for persistent or refractory NHL have increased the incidence of t-MNs [4]. Thus, t-MNs have become an important cause of morbidity and mortality in patients with indolent lymphomas [2].
With the prolonged survival of patients with indolent lymphomas, the co-emergence of t-MNs could be more frequent. Here, we describe a patient with coexistence of bone marrow (BM)-involved refractory marginal zone B-cell lymphoma (MZL) and t-MDS, who was treated with allogeneic hematopoietic stem cell transplantation (HSCT).
A 54-yr-old man visited the outpatient clinic of our hospital in November 2007 with multiple neck masses and weight loss. Computed tomography (CT) scanning confirmed multiple lymphadenopathies in the bilateral supraclavicular, lower neck, axillary, mediastinal, and retroperitoneal regions. Excisional biopsy of the cervical lymph node revealed MZL, the leukemic manifestation of which was confirmed by BM examination (32% of neoplastic lymphoid cells in the differential count of BM aspirates). Chromosomal analysis of the BM aspirate was performed not at the diagnosis of leukemic manifestation of MZL but at the time of first follow-up after starting the chemotherapy. At that time, BM examination revealed the persistence of leukemic manifestation of MZL, and chromosomal analysis showed a normal karyotype. After eight cycles of R-CVP chemotherapy consisting of rituximab, cyclophosphamide, vincristine, and prednisolone, and additional gemcitabine chemotherapy, the patient reached stable disease. The patient then received six cycles of FND chemotherapy composed of fludarabine, mitoxantrone, and dexamethasone, and was enrolled in the oxaliplatin clinical trial.
In July 2013, the patient was admitted with recurrent lymphoma of the abdomen, as revealed by CT scan, and sustained pancytopenia (leukocyte 2.5×109/L, hemoglobin 66 g/L, platelet 64×109/L). Although the BM aspirate was diluted, it showed the presence of neoplastic lymphoid cells (4.0% among nucleated cells), an increased number of myeloblasts (11.4%) (Fig. 1A), dyserythropoiesis (basophilic stippling and nuclear budding), and dysgranulopoiesis (abnormally lobulated nuclei, etc.). The BM biopsy showed multiple large nodules composed of small neoplastic B-lymphoid cells with CD20 positivity by immunohistochemical staining. In addition, the immunohistochemical stains for CD34 and CD117 revealed interstitially increased myeloblasts and immature myeloid cells (Fig. 1B-E). Chromosomal analysis of the BM aspirate showed a complex karyotype of monosomy 7, 44,XY,del(5)(q13;q35),-7,dic(12;20)(p13;q11.2),der(15)t(15;17)(p11.2;q11.2),-17,-18,+2mar [20]. The patient was diagnosed with BM involvement of MZL in progression and t-MDS. According to the revised International Prognostic Scoring System for MDS [5], he was classified into the very high-risk category. After 3 months, he received allogeneic HSCT from an unrelated donor, following a reduced-intensity conditioning (RIC) regimen consisting of fludarabine, melphalan, and antithymocyte globulin (ATG). On the 30th day post-HSCT, a follow-up BM assessment revealed normocellular marrow with trilineage regeneration without the evidence of BM involvement of lymphoma and a normal karyotype. Short tandem repeat analysis with peripheral blood on the 180th day post-HSCT revealed complete donor chimerism. The complete blood count showed a sustained hemoglobin level of more than 130 g/L and platelet count of more than 100×109/L without transfusion since the 96th day post-HSCT. He developed chronic graft-versus-host disease of the hepatic type, which improved after mycophenolate therapy. One year after HSCT, CT scanning revealed a decrease in the size of his enlarged lymph nodes. The patient has been stable for 21 months without evidence of recurrence.
Chemotherapeutic agents and ionizing radiation are well-known carcinogens, and WHO introduced t-MNs (including t-AML and t-MDS) as a new entity of disorders related to such therapies [6]. The well-recognized leukemogenic regimens are alkylating agents and topoisomerase II inhibitors, whereas other compounds such as platinum drugs are not clearly established [78]. The latency period from the first exposure to onset of t-MNs varies. It is generally known that t-MNs secondary to alkylating agents appear 4 to 7 yr after the exposure, whereas t-MNs secondary to topoisomerase II inhibitors appear after the shorter period of 1 to 5 yr [36]. The chemotherapeutic regimen of our patient included the alkylating agent cyclophosphamide, and it took 67 months from the time of exposure to chemotherapy until the development of t-MDS. Alkylating agents or radiotherapy typically cause t-MDS and induce chromosomal abnormalities involving chromosomes 5 (-5,del(5q)) and 7 (-7,del(7q)) [38]. Our patient showed a complex karyotype, including monosomy 7. In contrast, t-MN secondary to topoisomerase II inhibitors is associated with 11q23 or 21q22 abnormalities [3].
t-MDS accounts for about 20% of the MDS and is an independent adverse prognostic factor [18]. Treatment options for t-MDS are limited, and allogeneic RIC-HSCT is a curative option [3]. NHL is the most common hematologic malignancy in Korea, and MZL accounts for about 17% of Korean B-cell lymphoma cases [910]. All subtypes of MZL, classified as an indolent lymphoma, are highly responsive to rituximab, but there is no cure for patients with systemic spreading [11]. There have been some reports on the coexistence of BM-involved refractory lymphoma and t-MDS [312]. In a study of 17 patients with lymphoid malignancy and concurrent t-MDS, the median relapse-free survival and median overall survival was 4.3 months and 6.2 months, respectively [12]. Considering the history of extensive cytotoxic chemotherapy, frequent poor-risk cytogenetics, and recurrent preceding malignancy, allogeneic HSCT remains the only curative treatment option for patients with refractory lymphoma and concurrent t-MDS [312]. There is no established consensus on a conditioning regimen, but an RIC regimen consisting of fludarabine, melphalan, and ATG was used in the previous reports and in our patient [3].
The simultaneous presence of refractory lymphoma and t-MN in the BM is rare. To the best of our knowledge, this is the first report in Korea. Our patient has remained free from both malignancies for 21 months after allogeneic HSCT following RIC regimen. BM examination is necessary for restaging of the lymphoma to assess the response to therapy, or doing a workup for pancytopenia. Because the early diagnosis of t-MN is critical, it is important to closely examine BM specimens of patients who have undergone cytotoxic therapy, considering the potential for the coexistence of t-MNs.
Figures and Tables
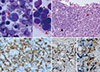 | Fig. 1Bone marrow (BM) findings of coexistence of BM-involved refractory marginal zone B-cell lymphoma and therapy-related myelodysplastic syndrome. (A) Neoplastic lymphoid cells (black arrow) and increased number of myeloblasts (red arrow) (BM aspirate, Wright stain, ×1,000). (B) Nodular infiltration of lymphoma cells (black arrow) and an area with increased number of myeloblasts (red arrow) (BM biopsy, H & E stain, ×400). (C) CD20-positive lymphoma cells in a lymphoid nodule (BM biopsy, immunohistochemistry for CD20, ×1,000). (D) Increased number of CD34-positive myeloblasts (BM clot section, immunohistochemistry for CD34, ×400). (E) Increased number of immature myeloid cells (BM clot section, immunohistochemistry for CD117, ×400). |
References
1. Larson RA. Cytogenetics, not just previous therapy, determines the course of therapy-related myeloid neoplasms. J Clin Oncol. 2012; 30:2300–2302.

2. Friedberg JW. Secondary malignancies after therapy of indolent non-Hodgkin’s lymphoma. Haematologica. 2008; 93:336–338.

3. Shimura Y, Kuroda J, Sasaki N, Uchiyama H, Ohshiro M, Matsumura Y, et al. Reduced-intensity allogeneic stem cell transplantation for co-emergence of chemotherapy-refractory follicular lymphoma and therapy-related myelodysplastic syndrome. Case Rep Oncol. 2014; 7:188–194.

4. Morton LM, Dores GM, Tucker MA, Kim CJ, Onel K, Gilbert ES, et al. Evolving risk of therapy-related acute myeloid leukemia following cancer chemotherapy among adults in the United States, 1975-2008. Blood. 2013; 121:2996–3004.

5. Greenberg PL, Tuechler H, Schanz J, Sanz G, Garcia-Manero G, Solé F, et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood. 2012; 120:2454–2465.

6. Zhang L. A focused review of hematopoietic neoplasms occurring in the therapy-related setting. Int J Clin Exp Pathol. 2014; 7:3512–3523.
7. Niscola P, Catalano G, Tendas A, Giovannini M, Scaramucci L, Neri B, et al. Complex and multifaceted therapy-related myeloid neoplasm following laryngeal cancer treated with Cisplatin and radiotherapy. Mediterr J Hematol Infect Dis. 2013; 5:e2013030.

8. Candelaria M, Dueñas-Gonzalez A. Therapy-related myelodysplastic syndrome. Expert Opin Drug Saf. 2015; 14:655–665.

9. Kang HJ, Kim WS, Kim SJ, Lee JJ, Yang DH, Kim JS, et al. Phase II trial of rituximab plus CVP combination chemotherapy for advanced stage marginal zone lymphoma as a first-line therapy: consortium for Improving Survival of Lymphoma (CISL) study. Ann Hematol. 2012; 91:543–551.

10. Park HJ, Park EH, Jung KW, Kong HJ, Won YJ, Lee JY, et al. Statistics of hematologic malignancies in Korea: incidence, prevalence and survival rates from 1999 to 2008. Korean J Hematol. 2012; 47:28–38.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download