Abstract
Background
Rotavirus is the leading cause of acute viral gastroenteritis, particularly in children, and is transmitted through the fecal-to-oral route by contaminated food or the environment. This study examined the contamination of the inner surfaces of domestic refrigerators with pathogens causing gastroenteritis.
Methods
Swab specimens from shelf surfaces of freezers and refrigerators were collected from 10 domestic refrigerators. Multiplex PCR for bacterial and viral pathogens causing acute gastroenteritis was performed. The VP7 and VP4 genes of rotavirus were amplified and then analyzed by DNA sequencing.
Results
Rotavirus was detected in five domestic refrigerators in the same apartment complex. All rotavirus samples showed the G1 genotype and the same DNA sequences. No pathogens causing acute gastroenteritis were identified in the other five domestic refrigerators.
Conclusions
The inner surfaces of domestic refrigerators can be contaminated with pathogens causing acute gastroenteritis, such as rotavirus. Attention should be given to the hygiene of refrigerators. To estimate the contamination or hygienic status for food storage, testing for viral pathogens combined with ordinary bacterial cultures may be necessary.
Figures and Tables
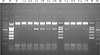 | Fig. 1Agarose gel electrophoresis results of multiplex PCR for viruses causing acute gastroenteritis. Lanes 1-5 represent each of five domestic refrigerators. Lowercase a and b represent freezer shelf and refrigerator shelf, respectively. Bands in lanes 2, 3, and 4 show positive results for rotavirus. The uppermost bands are internal controls.Abbreviations: M, marker; P, positive control; N, negative control.
|
References
1. Tate JE, Burton AH, Boschi-Pinto C, Steele AD, Duque J, Parashar UD, et al. 2008 estimate of worldwide rotavirus-associated mortality in children younger than 5 years before the introduction of universal rotavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis. 2012; 12:136–141.

2. Kim J, Kim HS, Kim HS, Kim JS, Song W, Lee KM, et al. Evaluation of an immunochromatographic assay for the rapid and simultaneous detection of rotavirus and adenovirus in stool samples. Ann Lab Med. 2014; 34:216–222.

3. Boone SA. Significance of fomites in the spread of respiratory and enteric viral disease. Appl Environ Microbiol. 2007; 73:1687–1696.

4. Barker J, Stevens D, Bloomfield SF. Spread and prevention of some common viral infections in community facilities and domestic homes. J Appl Microbiol. 2001; 91:7–21.

5. Butz AM, Fosarelli P, Dick J, Cusack T, Yolken R. Prevalence of rotavirus on high-risk fomites in day-care facilities. Pediatrics. 1993; 92:202–205.

6. Bloomfield SF. Cross-contamination and infection in the domestic environment and the role of chemical disinfectants. J Appl Microbiol. 1997; 83:1–9.

7. Kim JS, Kim HS, Hyun J, Kim HS, Song W, Lee KM, et al. Analysis of rotavirus genotypes in Korea during 2013: an increase in the G2P[4] genotype after the introduction of rotavirus vaccines. Vaccine. 2014; 32:6396–6402.

8. Agócs MM, Serhan F, Yen C, Mwenda JM, de Oliveira LH, Teleb N, et al. WHO global rotavirus surveillance network: a strategic review of the first 5 years, 2008-2012. MMWR Morb Mortal Wkly Rep. 2014; 63:634–637.
9. WHO. Manual of rotavirus detection and characterization methods. Geneva, Switzerland: World Health Organization;2009.
10. Han TH, Kim SC, Kim ST, Chung CH, Chung JY. Detection of norovirus genogroup IV, klassevirus, and pepper mild mottle virus in sewage samples in South Korea. Arch Virol. 2014; 159:457–463.

11. Gallimore CI, Taylor C, Gennery AR, Cant AJ, Galloway A, Xerry J, et al. Contamination of the hospital environment with gastroenteric viruses: comparison of two pediatric wards over a winter season. J Clin Microbiol. 2008; 46:3112–3115.

12. Carducci A, Verani M, Lombardi R, Casini B, Privitera G. Environmental survey to assess viral contamination of air and surfaces in hospital settings. J Hosp Infect. 2011; 77:242–247.

13. Dennehy PH. Transmission of rotavirus and other enteric pathogens in the home. Pediatr Infect Dis J. 2000; 19:S103–S105.

14. Lee C, Kim SJ. Molecular detection of human enteric viruses in urban rivers in Korea. J Microbiol Biotechnol. 2008; 18:1156–1163.
15. Park SH, Kim EJ, Yun TH, Lee JH, Kim CK, Seo YH, et al. Human enteric viruses in groundwater. Food Environ Virol. 2010; 2:69–73.

16. Cheong S, Lee C, Song SW, Choi WC, Lee CH, Kim SJ. Enteric viruses in raw vegetables and groundwater used for irrigation in South Korea. Appl Environ Microbiol. 2009; 75:7745–7751.

17. WHO. Five keys to safer food manual. Geneva, Switzerland: World Health Organization;2006.
18. Miko BA, Cohen B, Haxall K, Conway L, Kelly N, Stare D, et al. Personal and household hygiene, environmental contamination, and health in undergraduate residence halls in New York City, 2011. PLoS One. 2013; 8:e81460.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download