Abstract
Background
Since the concept of 'minimal identification of poor quality specimens or microbes with low pathogen potential' has been introduced into the standard operating procedure (SOP) to enhance work efficiency, consultations are requested for further species identification and antimicrobial susceptibility testing. The aim of this study was to evaluate the impact of consultations requests to the clinical microbiology laboratory on its work efficiency.
Methods
From January 2013 to April 2015, consultation requests to the laboratory in a tertiary-care hospital were collected from electronic medical records. The characteristics of consultations and changes to workflow due to the laboratory SOP amendment were analyzed. Turnaround time of the consultation and specimen culture were evaluated as an indicator of workflow efficiency.
Results
A total of 971 consultations were evaluated during the study period. The most common purposes for consultations were microbe species identification and antimicrobial susceptibility tests. Among the minimal identification reports, the proportions of consultations were below 5%. The number of consultations had increased substantially. However, the turnaround time of consultation and specimen culture showed declining trends.
Conclusions
With the introduction of the consultation system, the workload for species identification and antimicrobial susceptibility testing of colonizing microbes could be minimized. This research provides an example of work efficiency management for laboratory procedures based on an SOP amendment.
Figures and Tables
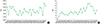 | Fig. 1Statistics of numbers of specimen culture and requested consultations during the study period. (A) Monthly numbers of requests for total culture specimens to the clinical microbiology laboratory for identification and antimicrobial sensitivity testing. (B) Number of consultation requests to the clinical microbiology laboratory for specific identification, initially reported as limited identification. |
 | Fig. 2Changing trends in the ratios of specimens reported as a positive culture result, limited identification, and further request for specific identification. |
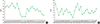 | Fig. 3Trends of turnaround time during the study period. (A) Changing turnaround time of specimen culture requests to the clinical microbiology laboratory. (B) Changing turnaround time of consultation requests to the clinical microbiology laboratory. |
Table 1
Reasons for consultation requests to the clinical microbiology laboratory

Table 2
Classification of consultation requests to the clinical microbiology laboratory that could not be answered

References
1. Abbott M, Paulin H, Sidhu D, Naugler C. Laboratory tests, interpretation, and use of resources: a program to introduce the basics. Can Fam Physician. 2014; 60:e167–e172.
3. Garcia , et al. Clinical microbiology procedures handbook. 3rd ed. Washington DC: ASM Press;2010.
4. Lyu SM, Byun JY, Choi YW, Choi HY. Clinical features of dermatology-consulted inpatients - focus on the differences between individual departments. Korean J Dermatol. 2014; 52:215–221.
5. Lee HY, Go SE, Son SH, Kim MK, Lee GH, Hyun MS. Inpatient consultations by the hematology department over a 1-year period. Korean J Med. 2009; 76:578–583.
6. Clinical and Laboratory Standards Institute. Performances standards for antimicrobial susceptibility testing. Twenty-fiftht Informational supplement, M100-S25. Wayne, PA: Clinical and Laboratory Standards Institute;2015.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download