Abstract
Background
We aimed to evaluate the performance of the CLINITEK Novus urine chemistry analyzer (Siemens, UK).
Methods
The precision, correlation, and carryover study were performed using two kinds of commercial quality control materials and 40-55 freshly collected patient specimens. We calculated exact and within-1-block agreement, along with kappa agreement, to compare the semi-quantitative results between urine chemistry analyzers. The urine specific gravity taken by a refractometer was compared with the analyzer results. Moreover, we analyzed additional urine specimens for protein to evaluate the agreement of results between those of the CLINITEK Novus and the AU680 analyzers (Beckman Coulter, Japan).
Results
The precision study showed acceptable results; within-1-block agreement was 100% in all tested items. The urine chemistry results from the CLNITEK Novus analyzer demonstrated ≥85.1% within-1-block agreements with those of the Uriscan Super, and the kappa test results were ≥0.81. The comparison of specific gravity with manual refractometer showed a good correlation (r=0.991), and the protein comparison with the AU680 analyzer also showed a good correlation (with exact and within-1-block agreements being 75.9% and 100.0%, respectively). The carryover rates were 0% in all tested items, except specific gravity and heavy blood tests.
Figures and Tables
 | Fig. 1Agreement and correlation of specific gravity between (A) the CLINITEK Novus analyzer and a refractometer, (B) Uriscan Super and a refractometer, (C) CLINITEK Novus and Uriscan Super. |
Table 1
Semiquantitative level grading criteria for the CLINITEK Novus and Uriscan Super analyzers according to their package inserts

Table 2
Precision analyzed by the CLINITEK Novus analyzer using CLINITEK Novus Control Strips and Liquichek Urinalysis Controls for 11 test parameters (N=20)

Table 3
Precision of specific gravity analyzed by the CLINITEK Novus analyzer

Table 4
Agreement percentages and kappa values of protein, glucose, ketone, occult blood, leukocytes, and pH using the CLINITEK Novus and Uriscan Super analyzers
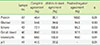
Table 5
Agreement of urine protein between the CLINITEK Novus analyzer and quantitation by the AU680 analyzer (grade, N=29)

| CLINITEK Novus | AU680* | |||||
|---|---|---|---|---|---|---|
| Negative | ± | 1+ | 2+ | 3+ | 4+ | |
| Negative | 6 | 1 | ||||
| ± | 3 | |||||
| 1+ | 5 | 1 | ||||
| 2+ | 2 | 4 | 2 | |||
| 3+ | 4 | 1 | ||||
| 4+ | ||||||
Complete agreement: 75.9%; Within-1-block agreement: 100.0%; Positive/negative agreement: 100.0%; Kappa test: 0.94. *Protein concentrations measured by the AU680 analyzer were graded according to the dipstick package insert (Siemens, NY, USA), grade (equivalent concentration); Negative, ±, 1+(30 mg/dL), 2+(100 mg/dL), 3+(300 mg/dL), 4+(2,000 mg/dL).
References
1. Kim KD. Urinalysis. In : Kang , editor. The Korean Society for Laboratory Medicine. Laboratory medicine. 5th ed. Seoul: E*PUBLIC;2014. p. 497–508.
2. McPherson RA, Ben-Ezra J. Basic examination of the urine. In : Henry JB, editor. Clinical diagnosis and management by laboratory methods. 22nd ed. Philadelphia: WB Saunders;2011. p. 445–479.
3. Han TH. Urinalysis: the usefulness and limitations of urine dipstick testing. J Korean Soc Pediatr Nephrol. 2013; 17:42–48.

4. Park HR, Jeong BK, Park NW. Comparative evaluation of dipstick urinalysis by dipstick readers. J Clin Pathol Qual Control. 2001; 23:239–246.
5. Clinical and Laboratory Standards Institute. User verification of precision and estimation of bias; Approved guideline-Third Edition. EP15-A3. Third Edition. Wayne, PA: Clinical and Laboratory Standards Institute;2014.
6. Clinical Laboratory Standards Institute. Urinalysis; Approved Guideline-Third Edition. GP16-A3. Wayne, PA: Clinical Laboratory Standards Institute;2012.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download