Abstract
Rhodotorula species are round to oval-shaped, multilateral budding, encapsulated yeasts that produce urease and do not ferment carbohydrates. Rhodotorula species form characteristic salmon-pink colored colonies owing to carotenoid pigment production. These yeasts form a part of the normal flora of moist skin and are found in the environment. Rhodotorula was traditionally considered a contaminant but is now progressively recognized as a human pathogen, especially in immunocompromised patients with central venous catheters. However, isolation of Rhodotorula species from blood has been very rarely reported in Korea. We report a case of sepsis due to Rhodotorula mucilaginosa infection in a patient who had received chemotherapy and supportive care for non-small cell lung cancer.
Rhodotorula species are known as contaminants because of their omnipresence in the environment such as in the air, soil, lakes, and ocean water. Due to their ubiquitous and saprophytic nature, the isolation of Rhodotorula from nonsterile sites such as the skin, sputum, urine, and feces has often been of doubtful clinical significance. However, it has been considered as pathogen, particularly in patients with leukemia or other solid tumors [1] and there were two cases of Rhodotorula bacteremia in Korea [23].
A 77-yr-old woman presented to the hospital with cough and dyspnea. Two years ago, she was diagnosed with non-small cell lung cancer and had been treated with wedge resection and several rounds of combined chemotherapy and radiation therapy.
In the present visit, her initial diagnosis was bronchopneumonia with pleural effusion. Empirical antibiotic therapy by intravenous piperacillin/sulbactam was started. She was stable and afebrile during the intravenous antibiotic treatment. Findings of computed tomography of the chest and abdomen suggested multiple metastases in the liver, lymph nodes in the lesser omentum, and left supraclavicular lymph nodes.
On day 22 of hospitalization, she developed fever of up to 38.1℃ without any evident source and her vital signs and laboratory findings were as follows: pulse rate, 90/min; respiratory rate, 20/min; blood pressure, 140/80 mmHg; leukocyte count, 12,550/mm3 (neutrophils: 83.5%); Hb, 11.5g/dL; platelet count, 137,000/mm3; erythrocyte sedimentation rate (ESR), 63 mm/hr; and C-reactive protein (CRP) level, 43.08 mg/L. Two peripheral blood samples were drawn and cultured. Based on the culture results, the antibiotic regimen was changed to meropenem and arbekacin. Within 1 day of initiating the new regimen, the patient's fever subsided and she remained afebrile. On day 27 of hospitalization, the patient again developed a fever of up to 38.3℃. Two sets of peripheral blood samples were cultured, and the findings were as follows: pulse rate, 110/min; respiratory rate, 20/min; blood pressure, 140/85 mmHg; leukocyte count, 8,500/mm3 (neutrophils: 86.2%); ESR, 57 mm/hr; and CRP level, 38.23 mg/L. However, her condition worsened and she died on day 34 of hospitalization.
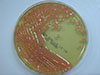
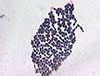
Aerobic cultures of blood samples drawn during the first episode of fever flagged positive 5 days after incubation; these were subcultured. The isolates gave rise to smooth, mucoid, glistening, pink to coral red colonies (Fig. 1). Gram staining revealed globose and elongated budding yeasts without any pseudohyphae (Fig. 2). The Vitek2 system (BioMerieux Inc., Hazelwood, USA) identified the isolated colonies as Rhodotorula glutinis/mucilaginosa (90% probability). Therefore, we performed molecular identification by sequencing the internal transcribed spacer (ITS) sequence of the ribosomal transcript. The ITS region was amplified using the primer sets ITS1 (5'-TCCGTAGGTGAACCTGCGG-3') with ITS1-F (3'-CTTGGTCATTTAGAGGAAGTAA-5') and ITS4 (5'-TCCTCCGCTTATTGATATGC-3') with ITS4-B (3'-CAGGAGACTTGTACACGGTCCAG-5'). The amplicons showed 98.85% similarity with the ITS region of R. mucilaginosa (NR_073296.1), confirming that the isolated fungus was R. mucilaginosa.
In addition, aerobic cultures of blood samples collected during the second episode of fever were also positive after 4 days of incubation, and the same microorganisms were identified. Antifungal susceptibility tests revealed that the isolate was susceptible to amphotericin B (minimum inhibitory concentration, MIC 0.5 µg/mL) and voriconazole (MIC 1.0 µg/mL) and resistant to fluconazole (MIC 32 µg/mL), casopofungin (MIC >8 µg/mL), and micafungin (MIC >8 µg/mL).
Rhodotorula species are the fourth most common non-candida yeast isolated from clinical specimens (4.2% of 8,821 isolates) [4]. The first case of Rhodotorula fungemia was reported in 1960 by Louria et al. in a patient with endocarditis [5]. Since then, a number of Rhodotorula infections have been reported. Recent studies have showed that the incidence of Rhodotorula fungemia is from 0.5% to 2.3% in Europe [6] and USA [7].
The major risk factors of Rhodotorula infection are prolonged use of central venous catheters in patients with a hematologic or solid malignancy, prolonged use of corticosteroids and/or cytotoxic drugs [78], and long-term used of broad-spectrum antibiotics [7]. The most prevalent species was R. mucilaginosa, formerly known as R. rubra, followed by R. glutinis. Our patient had been treated with cytotoxic agents eight times and broad-spectrum antibiotics daily for two months before admission, but no catheter was used.
Since Rhodotorula is not a common cause of infection, there are no definite treatment guidelines for Rhodotorula fungemia. In many cases, patients have been treated effectively with removal of the indwelling catheter and/or appropriate antifungal agents [237]. One in vitro study revealed that Rhodotorula species were reliably susceptible to amphotericin and flucytosine, but resistant to fluconazole and echinocandin; and susceptibility to new triazoles such as itraconazole, voriconazole and posconazole is not predictable [910]. The presumed mechanism of resistance to echinocandin is inhibition of access to the site of drug action in the fungal cell wall [11]. Although Rhodotorula species are less virulent than Candida and Cryptococcus species, the associated mortality rate was 12.6% in the largest systematic review [12]. In the current case, no catheter was inserted and only peripheral IV line was used. Before the antifungal susceptibility test, fluconazole was empirically administered two days after the second Rhodotorula isolation. However, given the high resistance to fluconazole and poor activity of echinocandin, amphotericin B may have been more beneficial in the current case. In contrast, there have been two reports of successful fluconazole treatment of Rhodotorula fungemia in cases in which fluconazole resistance was observed in vitro [1314].
In summary, we reported a case of R. mucilaginosa septicemia in a patient with advanced NSCLC in which long-term administration of broad-spectrum antibiotics may have contributed to this opportunistic infection. This is the first report of non-catheter-related sepsis due to R. mucilaginosa in Korea. Currently, amphotericin B is recommended as first-line therapy for Rhodotorula infection, but further evaluation of the effectiveness of new triazoles (e.g., voriconazole) based on antifungal susceptibility tests are required to determine appropriate therapy. Furthermore, correct and rapid identification of Rhodotorula species with antifungal susceptibility tests is also necessary.
Figures and Tables
References
1. El-Tahawy AT, Khalaf RM. Rhodotorula rubra fungemia in an immunocompromised patient. Ann Saudi Med. 1999; 19:533–535.

2. Chung JW, Kim BN, Kim YS. Central venous catheter-related Rhodotorula rubra fungemia. J Infect Chemother. 2002; 8:109–110.
3. Kim HA, Hyun M, Ryu SY. Catheter-associated Rhodotorula mucilaginosa fungemia in an immunocompetent host. Infect Chemother. 2013; 45:339–342.

4. Miceli MH, Diaz JA, Lee SA. Emerging opportunistic yeast infections. Lancet Infect Dis. 2011; 11:142–151.

5. Louria DB, Greenberg SM, Molander DW. Fungemia caused by certain nonpathogenic strains of the family Cryptococcaceae. Report of two cases due to Rhodotorula and Torulopsis glabrata. N Engl J Med. 1960; 263:1281–1284.

6. De Almeida GM, Costa SF, Melhem M, Motta AL, Szeszs MW, Miyashita F, et al. Rhodotorula spp. isolated from blood cultures: clinical and microbiological aspects. Med Mycol. 2008; 46:547–556.

7. Lunardi LW, Aquino VR, Zimerman RA, Goldani LZ. Epidemiology and outcome of Rhodotorula fungemia in a tertiary care hospital. Clin Infect Dis. 2006; 43:e60–e63.
8. Wirth F, Goldani LZ. Epidemiology of Rhodotorula: an emerging pathogen. Interdiscip Perspect Infect Dis. 2012; 2012:465717.
9. Diekema DJ, Petroelje B, Messer SA, Hollis RJ, Pfaller MA. Activities of available and investigational antifungal agents against Rhodotorula species. J Clin Microbiol. 2005; 43:476–478.

10. Zaas AK, Boyce M, Schell W, Lodge BA, Miller JL, Perfect JR. Risk of fungemia due to Rhodotorula and antifungal susceptibility testing of Rhodotorula isolates. J Clin Microbiol. 2003; 41:5233–5235.

11. Feldmesser M, Kress Y, Mednick A, Casadevall A. The effect of the echinocandin analogue caspofungin on cell wall glucan synthesis by Cryptococcus neoformans. J Infect Dis. 2000; 182:1791–1795.

12. Tuon FF, Costa SF. Rhodotorula infection. A systematic review of 128 cases from literature. Rev Iberoam Micol. 2008; 25:135–140.
13. Hsueh PR, Teng LJ, Ho SW, Luh KT. Catheter-related sepsis due to Rhodotorula glutinis. J Clin Microbiol. 2003; 41:857–859.

14. Sood S, Nerurkar V. Rhodotorula glutinis fungaemia. Indian Journal of Medical Specialities. 2013; 4:112–114.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download