Abstract
Background
Methods
Results
Figures and Tables
 | Fig. 1Line and scatter plots of stability test. X axis means elapsed time and Y axis means the average of % biases compared with the result of first measurement (0 hr) of each tube. The dashed lines mean upper and lower limits of desirable bias. (A) whole blood count (WBC), (B) hemoglobin (Hb), (C) platelet (PLT), (D) reticulocyte, (E) hemoglobin A1c (HbA1c), (F) brain natriuretic peptide (BNP), (G) prothrombin time (PT), (H) activated partial thrombin time (aPTT). |
Table 1
Information about evaluation tubes and control tubes, and measurands

Abbreviations: WBC, white blood cell; RBC, red blood cell; Hb, hemoglobin; HCT, hematocrit; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; RDW, red cell distribution width; PLT, platelet; MPV, mean platelet volume; PDW, platelet distribution width; PCT, platelet crit; HbA1c, hemoglobin A1c; BNP, brain natriuretic peptide; PT, prothrombin time; aPTT, activated partial thromboplastin time.
Table 2
Summary of evaluation methods

Table 3
Precision of Ampulab and BD tubes; the desirable precisions, measured total precisions of Ampulab and Vacutainer tubes, and decisions

*Instrument A includes XE-2100, Variant II Turbo, CA-7000; Instrument B includes ADVIA 2120i, Integra 800, STA compact; †Desirable precision is based on Westgard's biological database; ‡Measured precision means measured total precision of 3 lots of Ampulab tubes or 2 lots of Vacutainer tubes; §'Desirable' means measured total precision ≤desirable precision; 'Allowable' means measured total precision ≤95% confidential interval of desirable; precision; 'Not Allowable' means measured total precision >95% confidential interval of desirable precision.
Table 4
Accuracy of Ampulab tubes; desirable bias and measured bias of Ampulab tubes compared with Vacutainer tubes

*Instrument A includes XE-2100, Variant II Turbo, CA-7000; Instrument B includes ADVIA 2120i, Integra 800, STA compact; †Desirable bias is based on Westgard's biological database and presented absolute value; §R means correlation coefficient between the results of Ampulab tubes and the results of Vacutainer tubes; ∥'Desirable' means the estimated bias is within the desirable bias at each decision point; 'Allowable' means 95% confidential interval of the estimated bias overlaps with the desirable bias; 'Not Allowable' means 95% confidential interval of the estimated bias pass over the desirable bias; ¶BNP, exceptionally presented in this table, was measured by ADVIA Centaur XP as EDTA plasma test.
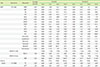
Table 5
Stability of Ampulab and Vacutainer tubes on 1st, 2nd, 3rd, 4th measurements (% change compared with 0 hr)




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download