Abstract
Background
We evaluated the efficacy of two chemiluminescence immunoassays that detect treponemal antibodies, Centaur Syphilis and Immulite Syphilis, in comparison with Mediace Treponema pallidum latex agglutination (TPLA).
Methods
The study was conducted in two phases. In the first phase, we tested 1,147 serum samples that were sequentially submitted for routine syphilis serology. In the second phase, we tested a panel of 119 frozen serum samples that had previously tested positive by Mediace RPR. The kappa value, total agreement percentage, and sensitivity and specificity were analyzed.
Results
Of the 1,147 random samples, 24 (2.09%) tested positive with Centaur Syphilis, 16 (1.39%) with Immulite Syphilis, and 19 (1.66%) with Mediace TPLA. Of the 119 Mediace RPR-positive samples, 103 (86.6%) tested positive with Centaur Syphilis, 101 (84.9%%) with Immulite Syphilis, and 105 (88.2%) with Mediace TPLA. The percent agreements (kappa values) were 98.8% (0.934) between Centaur Syphilis and Mediace TPLA, 99.0% (0.94) between Immulite Syphilis and Mediace TPLA, and 99.2% (0.955) between Centaur Syphilis and Immulite Syphilis. To measure the sensitivity and specificity of each treponemal test, samples showing agreement in three or four of the tests (three treponemal tests and Mediace-RPR) were regarded as true positive (n=117) or true negative (n=1,142). The respective values for sensitivity and specificity were 100% and 99.6% for Centaur Syphilis, 98.3% and 100% for Immulite Syphilis, and 99.2% and 99.7% for Mediace TPLA.
Figures and Tables
Table 1
Classification of sequentially RPR-requested serum samples (n=1,147) by reactivity to three treponemal assays, ADVIA Centaur Syphilis, Mediace TPLA, and IMMULITE 2000 Syphilis Screen

Table 2
Classification of Mediace RPR positive samples (n=119) based on reactivity to three treponemal assays, ADVIA Centaur Syphilis, Mediace TPLA, and IMMULITE 2000 Syphilis Screen
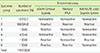
Table 3
Consensus classification of all samples (n=1,266) on the basis of the majority results for reactivity to three treponemal assays, ADVIA Centaur Syphilis, Mediace TPLA, and IMMULITE 2000 Syphilis Screen, and Mediace RPR

*The final classification of a specimen was positive when three treponemal assays showed reactivity, or two of three treponemal assays and Mediace RPR showed reactivity; the specimens were classified as negative when three treponemal assays showed non-reactivity, or two of three treponemal assays and Mediace RPR showed non-reactivity.
References
1. Larsen SA, Steiner BM, Rudolph AH. Laboratory diagnosis and interpretation of tests for syphilis. Clin Microbiol Rev. 1995; 8:1–21.

2. Choi KC. Recent trends in clinical observation of syphilis and consideration for laboratory tests. J Korean Med Assoc. 2009; 52:1100–1106.

3. Workowski KA, Berman S. Centers for Disease Control and Prevention (CDC). Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep. 2010; 59:1–110.
4. French P, Gomberg M, Janier M, Schmidt B, van Voorst Vader P, Young H. IUSTI. 2008 European Guidelines on the Management of Syphilis. Int J STD AIDS. 2009; 20:300–309.

5. Korean Centers for Disease Control and Prevention, The Korean Association of Urogenital Tract Infection and Inflammation. Sexually Transmitted Infection Korean Treatment Guideline.
6. Centers for Disease Control and Prevention (CDC). Syphilis testing algorithms using treponemal tests for initial screening--four laboratories, New York City, 2005-2006. MMWR Morb Mortal Wkly Rep. 2008; 57:872–875.
7. Centers for Disease Control and Prevention (CDC). Discordant results from reverse sequence syphilis screening-five laboratories, United States, 2006-2010. MMWR Morb Mortal Wkly Rep. 2011; 60:133–137.
8. Marangoni A, Moroni A, Accardo S, Cevenini R. Laboratory diagnosis of syphilis with automated immunoassays. J Clin Lab Anal. 2009; 23:1–6.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download