Abstract
Background
Cellular analysis of bronchoalveolar lavage fluid (BALF) is a useful diagnostic tool for interstitial lung diseases (ILDs). The lymphocytes in BALF consist of CD3+CD4+ T cells (T4), CD3+CD8+ T cells (T8), and a few B cells. However, sometimes, an increased number of CD3+CD4-CD8- T cells (double-negative T cells, DNTs) are noted in BALF. It is known that DNTs in the blood are associated with immunoregulation and autoimmune diseases. However, there are only few studies on DNTs in BALF. We evaluated the DNTs in BALF in patients with pulmonary diseases.
Methods
Immunophenotyping results of the BALF obtained from 122 pulmonary disease patients over an 8-yr period were reviewed. T-lymphocyte subsets (T4, T8, and DNT) and inflammatory markers were analyzed for each group of clinical diagnosis. T-lymphocyte percentage of more than 15% of the total cells was defined as BALF lymphocytosis, and DNT percentage of more than 5% of T lymphocytes was defined as high DNT.
Results
The most frequent diseases found in the patients were pneumonia (31.6%), autoimmune-related ILDs (18.0%), hypersensitivity pneumonitis (10.7%), and organizing pneumonia (10.7%). However, the occurrence of autoimmune-related ILDs was significantly high (40%) in patients with lymphocytosis and high DNT (P=0.002). All lung cancer patients showed lymphocytosis with high DNT. In addition, CD3-signal intensities of DNTs were significantly higher than those of other T-lymphocyte subtypes (P=0.003).
Figures and Tables
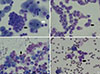 | Fig. 1Representation of nucleated cells in cytocentrifuged bronchoalveolar lavage fluid (Wright stain, ×600). (A) Lymphocytes (black arrow) and alveolar macrophages (white arrow); (B) Neutrophils (black arrow); (C) Eosinophils (black arrow); (D) Ciliated epithelial cells (black arrow). |
 | Fig. 2CD3 mean fluorescence intensity of double-negative T (DNT, green) was higher than that of T8 (blue, 10.54 vs. 8.03, P=0.001) and T4 subtypes (purple, 10.54 vs. 8.19, P=0.003). |
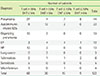
Table 1
Diagnosis and the results of BALF immunophenotyping of total 122 patients
References
1. Meyer KC, Raghu G. Bronchoalveolar lavage for the evaluation of interstitial lung disease: is it clinically useful? Eur Respir J. 2011; 38:761–769.

2. Wuyts WA, Dooms C, Verleden GM. The clinical utility of bronchoalveolar lavage cellular analysis in interstitial lung disease. Am J Respir Crit Care Med. 2013; 187:777.

3. Papiris SA, Kollintza A, Kitsanta P, Kapotsis G, Karatza M, Milic-Emili J, et al. Relationship of BAL and lung tissue CD4+ and CD8+ T lymphocytes, and their ratio in idiopathic pulmonary fibrosis. Chest. 2005; 128:2971–2977.

4. Caillaud DM, Vergnon JM, Madroszyk A, Melloni BM, Murris M, Dalphin JC, et al. Bronchoalveolar lavage in hypersensitivity pneumonitis: a series of 139 patients. Inflamm Allergy Drug Targets. 2012; 11:15–19.

5. Jara-Palomares L, Martin-Juan J, Gomez-Izquierdo L, Cayuela-Dominguez A, Rodriguez-Becerra E, Rodriguez-Panadero F. [Bronchoalveolar lavage findings in patients with diffuse interstitial lung disease: prospective study of a cohort of 562 patients]. Arch Bronconeumol. 2009; 45:111–117.

6. Capelozzi VL, Faludi EP, Balthazar AB, Fernezlian Sde M, Filho JV, Parra ER. Bronchoalveolar lavage improves diagnostic accuracy in patients with diffuse lung disease. Diagn Cytopathol. 2013; 41:1–8.

7. Picinin IF, Camargos PA, Marguet C. Cell profile of BAL fluid in children and adolescents with and without lung disease. J Bras Pneumol. 2010; 36:372–385.
8. Goldman L, Schafer AI. Respiratory structure and function. In : Reynolds H, editor. Goldman's Cecil Medicine. 24th ed. Saunders: Elsevier;2012. p. 523–527.
9. Hillhouse EE, Lesage S. A comprehensive review of the phenotype and function of antigen-specific immunoregulatory double negative T cells. J Autoimmun. 2013; 40:58–65.

10. Dean GS, Anand A, Blofeld A, Isenberg DA, Lydyard PM. Characterization of CD3+ CD4- CD8- (double negative) T cells in patients with systemic lupus erythematosus: production of IL-4. Lupus. 2002; 11:501–507.

11. Anand A, Dean GS, Quereshi K, Isenberg DA, Lydyard PM. Characterization of CD3+ CD4- CD8- (double negative) T cells in patients with systemic lupus erythematosus: activation markers. Lupus. 2002; 11:493–500.

12. Crispin JC, Oukka M, Bayliss G, Cohen RA, Van Beek CA, Stillman IE, et al. Expanded double negative T cells in patients with systemic lupus erythematosus produce IL-17 and infiltrate the kidneys. J Immunol. 2008; 181:8761–8766.

13. Crispin JC, Tsokos GC. IL-17 in systemic lupus erythematosus. J Biomed Biotechnol. 2010; 2010:943254.
14. Song KS, Heo WB, Won DI. [Comparative analysis of bronchoalveolar lavages in interstitial lung diseases]. Korean J Lab Med. 2007; 27:221–227.

15. Hyldgaard C, Kaae S, Riddervold M, Hoffmann HJ, Hilberg O. Value of s-ACE, BAL lymphocytosis, and CD4+/CD8+ and CD103+CD4+/CD4+ T-cell ratios in diagnosis of sarcoidosis. Eur Respir J. 2012; 39:1037–1039.

16. Su D, Shen M, Li X, Sun L. Roles of γδ T Cells in the pathogenesis of autoimmune diseases. Clin Dev Immunol. 2013; 2013:985753.
17. Lee AJ, Kim SG, Chae HD, Lee GH, Shin IH. γδ T cells are increased in the peripheral blood of patients with gastric cancer. Clin Chim Acta. 2012; 413:1495–1499.

18. Bakir HY, Tomiyama-Miyaji C, Watanabe H, Nagura T, Kawamura T, Sekikawa H, et al. Reasons why DBA/2 mice are resistant to malarial infection: expansion of CD3int B220+ gammadelta T cells with double-negative CD4- CD8- phenotype in the liver. Immunology. 2006; 117:127–135.

19. Liang Q, Jiao Y, Zhang T, Wang R, Li W, Zhang H, et al. Double Negative (DN) [CD3(+)CD4(-)CD8(-)] T cells correlate with disease progression during HIV infection. Immunol Invest. 2013; 42:431–437.

20. D'Acquisto F, Crompton T. CD3+CD4-CD8- (double negative) T cells: saviours or villains of the immune response? Biochem Pharmacol. 2011; 82:333–340.
21. Mixter PF, Russell JQ, Morrissette GJ, Charland C, Aleman-Hoey D, Budd RC. A model for the origin of TCR-alphabeta+ CD4-CD8- B220+ cells based on high affinity TCR signals. J Immunol. 1999; 162:5747–5756.
22. Fisher GH, Rosenberg FJ, Straus SE, Dale JK, Middleton LA, Lin AY, et al. Dominant interfering Fas gene mutations impair apoptosis in a human autoimmune lymphoproliferative syndrome. Cell. 1995; 81:935–946.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download