Abstract
Background
Lung cancer is the most lethal malignant neoplasm in the world. Serum cytokeratin fragment 21-1 (Cyfra 21-1) is a valuable tumor marker for detection of lung cancer, and it has good sensitivity and specificity. The aim of this study was to investigate the diagnostic value of Cyfra 21-1 levels in patients with lung cancer.
Methods
We retrospectively reviewed 814 samples from 169 patients with lung cancer, 124 patients with benign pulmonary diseases, and 521 normal controls from health check-up clinic. Serum Cyfra 21-1 levels were determined with Architect CYFRA 21-1 kit (Abbott, USA) using Architect i2000 analyzer.
Results
Median levels and interquartile ranges for Cyfra 21-1 in patients with lung cancer (non-small cell lung cancer: 3.16 [1.98, 9.00] ng/mL, small cell lung cancer: 3.32 [2.07, 5.20] ng/mL) were higher than those in patients with benign pulmonary diseases (1.50 [1.17, 2.17] ng/mL; P<0.01) and in normal controls (1.26 [0.93, 1.75] ng/mL; P<0.01). Sensitivity, specificity, positive predictive value, and negative predictive value for Cyfra 21-1 were 70.4%, 81.2%, 49.6%, and 91.3%, respectively. The area under the curve for Cyfra 21-1 was 0.839 (95% confidence interval, 0.802-0.877).
Figures and Tables
 | Fig. 1Receiver operating characteristic (ROC) curve using serum Cyfra 21-1 level to discriminate between the lung cancer and control groups (A) (AUC = 0.839 [95% CI, 0.802-0.877]) and the squamous cell cancer and control groups (B) (AUC = 0.866 [95% CI, 0.810-0.922]). |
Table 1
Patient characteristics
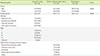
Table 2
Multivariate analysis for lung cancer patients

| Variable | Odds ratio | 95% confidence interval | P value |
|---|---|---|---|
| Sex | 0.961 | 0.542-1.707 | 0.893 |
| Age | 1.780 | 1.490-2.126 | < 0.001 |
| Cyfra 21-1 | 1.110 | 1.089-1.132 | < 0.001 |
Table 3
Cyfra 21-1 and CEA levels (median [interquartile range])

Table 4
Sensitivity, specificity, positive predictive value, and negative predictive value for Cyfra 21-1 and CEA in lung cancer diagnosis

References
1. Wang R, Wang G, Zhang N, Li X, Liu Y. Clinical evaluation and cost-effectiveness analysis of serum tumor markers in lung cancer. Biomed Res Int. 2013; 2013:195692.

2. Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010; 127:2893–2917.

3. Park HD, Jeong HS, Park JS, Lim SH, Lee EJ, Yun JW, et al. Usefulness of cyfra 21-1 as a tumor marker of lung cancer. Korean J Med. 2002; 62:415–421.
4. Park SY, Lee JG, Kim J, Park Y, Lee SK, Bae MK, et al. Preoperative serum CYFRA 21-1 level as a prognostic factor in surgically treated adenocarcinoma of lung. Lung Cancer. 2013; 79:156–160.

5. Stieber P, Bodenmüller H, Banauch D, Hasholzner U, Dessauer A, Ofenloch-Hähnle B, et al. Cytokeratin 19 fragments: a new marker for non-small cell lung cancer. Clin Biochem. 1993; 26:301–304.
6. Edelman MJ, Hodgson L, Rosenblatt PY, Christenson RH, Vokes EE, Wang X, et al. CYFRA 21-1 as a prognostic and predictive marker in advanced non-small cell lung cancer in a prospective trial: CALGB 150304. J Thorac Oncol. 2012; 7:649–654.

7. Lee S, Lee CY, Kim DJ, Hong DJ, Lee JG, Chung KY. Pathologic correlation of serum carcinoembryonic antigen and cytokeratin 19 fragment in resected nonsmall cell lung cancer. Korean J Thorac Cardiovasc Surg. 2013; 46:192–196.

8. Ono A, Takahashi T, Mori K, Akamatsu H, Shukuya T, Taira T, et al. Prognostic impact of serum CYFRA 21-1 in patients with advanced lung adenocarcinoma: a retrospective study. BMC Cancer. 2013; 13:354.

9. Chen Q, Ge F, Cui W, Wang F, Yang Z, Guo Y, et al. Lung cancer circulating tumor cells isolated by the EpCAM-independent enrichment strategy correlate with Cytokeratin 19-derived CYFRA21-1 and pathological staging. Clin Chim Acta. 2013; 419:57–61.

10. Jung M, Kim SH, Hong S, Kang YA, Kim SK, Chang J, et al. Prognostic and predictive value of carcinoembryonic antigen and cytokeratin-19 fragments levels in advanced non-small cell lung cancer patients treated with gefitinib or erlotinib. Yonsei Med J. 2012; 53:931–939.

11. Moll R, Franke WW, Schiller DL, Geiger B, Krepler R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell. 1982; 31:11–24.

12. Niklinski J, Furman M, Rapellino M, Chyczewski L, Laudanski J, Oliaro A, et al. CYFRA 21-1 determination in patients with non-small cell lung cancer: clinical utility for the detection of recurrences. J Cardiovasc Surg (Torino). 1995; 36:501–504.
13. Pujol JL, Grenier J, Daurès JP, Daver A, Pujol H, Michel FB. Serum fragment of cytokeratin subunit 19 measured by CYFRA 21-1 immunoradiometric assay as a marker of lung cancer. Cancer Res. 1993; 53:61–66.
14. Cho KJ, Lee KH, Lee JW, Song KE, Lee WK, Kim JS, et al. CYFRA 21-1, the new marker for lung cancer. Korean J Clin Pathol. 1997; 17:55–64.
15. Okamura K, Takayama K, Izumi M, Harada T, Furuyama K, Nakanishi Y. Diagnostic value of CEA and CYFRA 21-1 tumor markers in primary lung cancer. Lung Cancer. 2013; 80:45–49.

16. Yu H, Huang X, Zhu Z, Hu Y, Ou W, Zhang L, et al. Significance of combined detection of LunX mRNA and tumor markers in diagnosis of lung carcinoma. Chin J Cancer Res. 2014; 26:89–94.
17. Hatzakis KD, Froudarakis ME, Bouros D, Tzanakis N, Karkavitsas N, Siafakas NM. Prognostic value of serum tumor markers in patients with lung cancer. Respiration. 2002; 69:25–29.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download