Abstract
Background
Cardiac troponins I (cTnI) and T (cTnT) have received international endorsement as the standard biomarkers for detection of myocardial injury, for risk stratification in patients suspected of acute coronary syndrome, and for the diagnosis of myocardial infarction. An evidence-based clinical database is growing rapidly for high-sensitivity (hs) troponin assays. Thus, clarifications of the analytical principles for the immunoassays used in clinical practice are important.
Content
The purpose of this mini-review is (a) to provide a background for the biochemistry of cTnT and cTnI and (b) to address the following analytical questions for both hs cTnI and cTnT assays: (i) How does an assay become designated hs? (ii) How does one realistically define healthy (normal) reference populations for determining the 99th percentile? (iii) What is the usual biological variation of these analytes? (iv) What assay imprecision characteristics are acceptable? (v) Will standardization of cardiac troponin assays be attainable?
Summary
This review raises important points regarding cTnI and cTnT assays and their reference limits and specifically addresses hs assays used to measure low concentrations (nanograms per liter or picograms per milliliter). Recommendations are made to help clarify the nomenclature. The review also identifies further challenges for the evolving science of cardiac troponin measurement. It is hoped that with the introduction of these concepts, both laboratorians and clinicians can develop a more unified view of how these assays are used worldwide in clinical practice.
Go to : 
References
1. Thygesen K, Alpert JS, White HD, on behalf of the Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction. Universal definition of myocardial infarction. Eur Heart J. 2007; 28:2525–38.
2. Morrow DA, Cannon CP, Jesse RL, Newby LK, Ravkilde J, Storrow AB, et al. National Academy of Clinical Biochemistry practice guidelines: clinical characteristics and utilization of biomarkers in acute coronary syndromes. Clin Chem. 2007; 53:552–74.
3. Apple FS, Jesse RL, Newby LK, Wu AHB, Christenson RH. National Academy of Clinical Biochemistry and IFCC Committee for Standardization of Markers of Cardiac Damage laboratory medicine practice guidelines: analytical issues for biomarkers of acute coronary syndromes. Clin Chem. 2007; 53:547–51.
4. Jaffe AS, Apple FS. High-sensitive cardiac troponin: hype, help and reality. Clin Chem. 2010; 56:342–4.
5. Reichlin T, Irfan A, Twerenbold R, Reiter M, Hochholzer W, Burkhalter H, et al. Utility of absolute and relative changes in cardiac troponin concentrations in the early diagnosis of acute myocardial infarction. Circulation. 2011; 124:136–45.

6. de Lemos JA, Drazner MH, Omland T, Ayers CR, Khera A, Rohatgi A, et al. Association of troponin T detected with a highly sensitive assay and cardiac structure and mortality risk in the general population. JAMA. 2010; 304:2503–12.

7. de Flippi CR, de Lemos JA, Christenson RH, Gottdiener JS, Kop WJ, Zhan M, et al. Association of serial measures of cardiac troponin T using a sensitive assay with incident heart failure and cardiovascular mortality in older adults. JAMA. 2010; 304:2494–502.

8. Venge P, Johnston N, Lindahl B, James S. Normal plasma levels of cardiac troponin I measured by the high-sensitivity cardiac troponin I Access prototype assay and the impact on the diagnosis of myocardial ischemia. J Am Coll Cardiol. 2009; 54:1165–72.

9. Apple FS. A new season for cardiac troponin assays: It's time to keep a scorecard. Clin Chem. 2009; 55:1303–6.

10. Gaze DC, Collinson PO. Multiple molecular forms of circulating cardiac troponin: analytical and clinical significance. Ann Clin Biochem. 2008; 45:349–55.

11. Ricchiuti V, Voss EM, Ney A, Odland M, Anderson PA, Apple FS. Cardiac troponin T isoforms expressed in renal diseased skeletal muscle will not cause false-positive results by the second generation cardiac troponin T assay by Boehringer Mannheim. Clin Chem. 1998; 44:1919–24.

12. Jaffe AS, Vasile VC, Milone M, Saenger AK, Olson KN, Apple FS. Diseased skeletal muscle: a noncardiac source for increased circulating concentrations of cardiac troponin T. J Am Coll Cardiol. 2011; 58:1819–24.
13. Voss EM, Sharkey SW, Gernert AE, Murakami MM, Johnston RB, Hsieh CC, Apple FS. Human and canine cardiac troponin T and creatine kinase-MB distribution in normal and diseased myocardium. Infarct sizing using serum profiles. Arch Pathol Lab Med. 1995; 119:799–806.
14. White HD. Pathobiology of troponin elevations: Do elevations occur with myocardial ischemia as well as necrosis? J Am Coll Cardiol. 2011; 57:2406–8.
15. Katus HA, Remppis A, Scheffold T, Diederich KW, Kuebler W. Intracellular compartmentation of cardiac troponin T and its release kinetics in patients with reperfused and nonreperfused myocardial infarction. Am J Cardiol. 1991; 67:1360–7.

16. Wu AH, Feng YJ, Moore R, Apple FS, McPherson PH, Buechler KF, Bodor G, for the American Association for Clinical Chemistry Subcommittee for, and Clinical Chemistry Subcommittee on cTnI Standardization. Characterization of cardiac troponin subunit release into serum after acute myocardial infarction and comparison of assays for troponin T and I. Clin Chem. 1998; 44:1198–208.
17. Michielsen EC, Diris JH, Wodzig WK, Dieijen-Visser MP. Size-exclusion chromatography of circulating cardiac troponin T. Clin Chem. 2006; 52:2306–7.

18. Saenger AK, Beyrau R, Braun S, Cooray R, Dolci A, Freidank H, et al. Multicenter analytical evaluation of a high-sensitivity troponin T assay. Clin Chim Acta. 2011; 412:748–54.

19. Hallermayer K, Klenner D, Vogel R. Use of recombinant human cardiac troponin T for standardization of third generation troponin T methods. Scand J Clin Lab Invest Suppl. 1999; 230:128–31.

20. Katrukha A, Bereznikova A, Filatov V, Esakova T. Biochemical factors influencing measurement of cardiac troponin I in serum. Clin Chem Lab Med. 1999; 37:1091–5.

21. Adams JE, Bodor GS, Davila-Roman VG, Delmez JA, Apple FS, Ladenson JH, Jaffe AS. Cardiac troponin I: a marker with high specificity for cardiac injury. Circulation. 1993; 88:101–6.

22. Cummins B, Auckland ML, Cummins P. Cardiacspecific troponin-I radioimmunoassay in the diagnosis of acute myocardial infarction. Am Heart J. 1987; 113:1333–44.
23. Christenson RH, Duh SH, Apple FS, Bodor G, Bunk D, Dalluge J, et al. Standardization of cardiac troponin I assays: round robin performance of ten candidate reference materials. Clin Chem. 2001; 47:431–7.
24. Tate JR, Bunk DM, Christenson RH, Katrukha A, Noble JE, Porter RA, et al. Standardisation of cardiac troponin I measurement: past and present. Pathology. 2010; 42:402–8.

25. Katrukha AG, Bereznikova AV, Filatov VL, Esakova TV, Kolosova V, Pettersson K, et al. Degradation of cardiac troponin I: implication for reliable immunodetection. Clin Chem. 1998; 44:2433–40.

26. Katrukha AG, Bereznikova AV, Esakova TV, Pettersson K, Lovgren T, Severina ME, et al. Troponin I is released in bloodstream of patients with acute myocardial infarction not in free form but as complex. Clin Chem. 1997; 43:1379–85.
27. Eriksson S, Halenius H, Pulkki K, Hellman J, Pettersson K. Negative interference in cardiac troponin I immunoassays by circulating troponin autoantibodies. Clin Chem. 2005; 51:839–47.

28. HyTest Ltd. Troponins. http://www.troponins.com/troponins. (Accessed July 2011).
29. Giannitsis E, Kurz K, Hallermayer K, Jarausch J, Jaffe AS, Katus HA. Analytical validation of a high-sensitivity cardiac troponin T assay. Clin Chem. 2010; 56:254–61.

30. Collinson PO, Heung YM, Gaze D, Boa F, Senior R, Christenson R, Apple FS. Influence of population selection on the 99th percentile reference value for cardiac troponin assays. Clin Chem. 2012; 58:219–25.

31. de Lemos JA, McGuire DK, Khera A, Das SR, Murphy SA, Omland T, Drazner MH. Screening the population for left ventricular hypertrophy and left ventricular systolic dysfunction using natriuretic peptides: results from the Dallas Heart Study. Am Heart J. 2009; 157:746–53.

32. Apple FS, Simpson PA, Murakami MM. Defining the serum 99th percentile in a normal reference population measured by a high-sensitivity cardiac troponin I assay. Clin Biochem. 2010; 43:1034–6.

33. Apple FS, Murakami MM. Serum and plasma cardiac troponin I 99th percentile reference values for 3 2nd-generation assays. Clin Chem. 2007; 53:1558–60.

34. Collinson PO, Clifford-Mobley O, Gaze D, Boa F, Senior R. Assay imprecision and 99th-percentile reference value of a high-sensitivity cardiac troponin I assay. Clin Chem. 2009; 55:1433–4.

35. Wu AHB, Quynh AL, Todd J, Moecks J, Wians F. Short- and longterm biological variation in cardiac troponin I measured with a high-sensitivity assay: implications for clinical practice. Clin Chem. 2009; 55:528.

36. Vasile VC, Saenger AK, Kroning JM, Jaffe AS. Biological and analytical variability of a novel high-sensitivity cardiac troponin T assay. Clin Chem. 2010; 56:1086–90.

37. Frankenstein L, Wu AHB, Hallermayer K, Wians FH Jr, Giannitsis E, Katus HA. Biological variation and reference change value of high-sensitivity troponin T in healthy individuals during short and intermediate follow-up periods. Clin Chem. 2011; 57:1068–71.

38. Apple FS, Murakami MM, Wians FH, Ler R, Kaczmarek JM, Wu AHB. Short-term biological variation of cardiac troponin I measured with three high-sensitivity assays [Abstract]. Clin Chem. 2011; 57:C05.
39. Apple FS, Parvin CA, Buechler KF, Christenson RH, Wu AHB, Jaffe AS. Validation of the 99th percentile cutoff independent of assay imprecision (CV) for cardiac troponin monitoring for ruling out myocardial infarction. Clin Chem. 2005; 51:2198–200.

40. zKupchak P, Wu AHB, Ghani F, Newby K, Ohman EM, Christenson RH. Influence of imprecision on ROC curve analysis for cardiac markers. Clin Chem. 2006; 52:752–3.
Go to : 
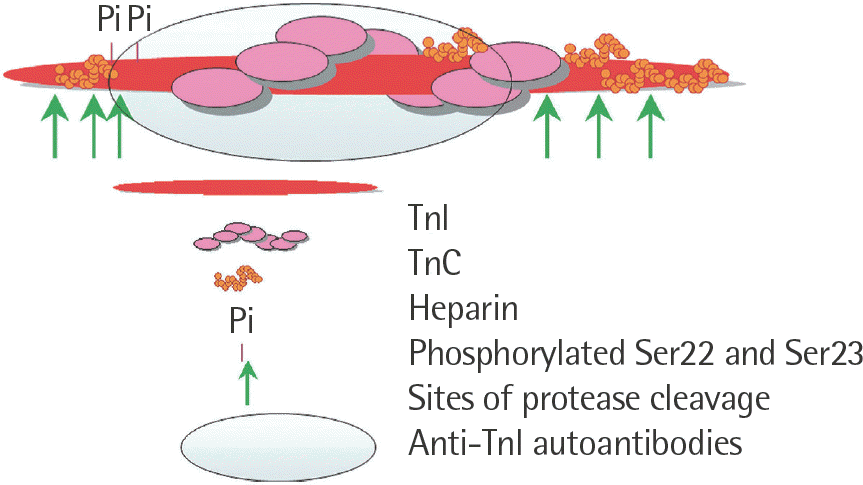 | Fig. 1.Cardiac troponin I epitopes that are prone to interference. Ada pted from HyTest Ltd. [28]. |
Table 1.
Analytical characteristics of contemporary sensitive and point-of-care cardiac troponin assays.
| Company/platform/assay | Cardiac troponin concentration at: | Amino acid residues of epitopes recognized by capture (C) and detection (D) MAbs | ||
|---|---|---|---|---|
| LoD (μg/L) | 99th Percentile (μg/L, CV)∗ | 10% CV concentration (μg/L) | ||
| Abbott AxSYM ADV | 0.02 | 0.04 (14) | 0.16 | C: 87–91, 41–49; D: 24–40 |
| Abbott ARCHITECT | 0.009 | 0.028 (14) | 0.032 | C: 87–91, 24–40; D: 41–49 |
| Abbott i-STAT | 0.02 | 0.08 (16.5) | 0.10 | C: 41–49, 88–91; D: 28–39, 62–78 |
| Alere Triage | 0.05 | <0.05 (NA) | NA | C: NA; D: 27–40 |
| Alere Triage Cardio3† | 0.01 | 0.02 (17) | NA | C: 27–39; D: 83–93, 190–196 |
| Beckman Access AccuTnI | 0.01 | 0.04 (14) | 0.06 | C: 41–49; D: 24–40 |
| bioMérieux Vidas Ultra | 0.01 | 0.01 (27.7) | 0.11 | C: 41–49, 22–29; D: 87–91, MAb 7B9 |
| Mitsubishi Pathfast | 0.008 | 0.029 (5.0) | 0.014 | C: 41–49; D: 71–116, 163–209 |
| Ortho Vitros ECi ES | 0.012 | 0.034 (10) | 0.034 | C: 24–40, 41–49; D: 87–91 |
| Radiometer AQT90 cTnI | 0.009 | 0.023 (17.7) | 0.039 | C: 41–49, 190–196; D: 137–149 |
| Radiometer AQT90 cTnT | 0.008 | 0.017 (15.2) | 0.026 | C: 125–131; D: 136–147 |
| Response RAMP | 0.03 | <0.01 (18.5 at 0.05) | 0.21 | C: 85–92; D: 26–38 |
| Roche cobas h232 Cardiac T†,‡ | 0.05 | NA | NA | C: 125–131; D: 136–147 |
| Roche Elecsys TnT Gen 4 | 0.01 | <0.01 | 0.030 | C: 136–147; D: 125–131 |
| Roche Elecsys TnI | 0.16 | 0.16 (10) | 0.30 | C: 87–91, 190–196; D: 23–29, 27–43 |
| Roche Cardiac Reader cTnT§ | 0.03 | NA | NA | C: 125–131; D: 136–147 |
| Siemens Centaur Ultra | 0.006 | 0.04 (8.8) | 0.03 | C: 41–49, 87–91; D: 27–40 |
| Siemens Dimension RxL | 0.04 | 0.07 (20) | 0.14 | C: 27–32; D: 41–56 |
| Siemens Immulite 2500 | 0.1 | 0.2 (NA) | 0.42 | C: 87–91; D: 27–40 |
| Siemens Stratus CS | 0.03 | 0.07 (10) | 0.06 | C: 27–32; D: 41–56 |
| Siemens Vista | 0.015 | 0.045 (10) | 0.04 | C: 27–32; D: 41–56 |
| Tosoh AIA | 0.06 | <0.06 (NA) | 0.09 | C: 41–49; D: 87–91 |
Table 2.
Analytical characteristics of hs cardiac troponin assays.
| Company/ platform/assay | Cardiac troponin concentration at: | Amino acid residues of epitopes recognized by capture (C) and detection (D) MAbs | ||
|---|---|---|---|---|
| LoD (ng/L) | 99th Percentile (ng/L, CV)∗ | 10% CV concentration (ng/L) | ||
| hs-cTnI | ||||
| Abbott ARCHITECT† | 1.2 | 16 (5.6) | 3.0 | C: 24–40; D: 41–49 |
| Beckman Access† | 2–3 | 8.6 (10) | 8.6 | C: 41–49; D: 24–40 |
| Nanosphere MTP† | 0.2 | 2.8 (9.5) | 0.5 | C: 136–147; D: MAb PA1010 |
| Singulex Erenna† | 0.09 | 10.1 (9.0) | 0.88 | C: 41–49; D: 27–41 |
| Siemens Vista† | 0.5 | 9 (5.0) | 3 | C: 30–35; D: 41–56, 171–190 |
| hs-cTnT | ||||
| Roche Elecsys‡ | 5.0 | 14 (8) | 13 | C: 136–147; D: 125–131 |
Table 3.
Short-term analytical and biological variation by hs-cTnI assays.
| Abbott∗ | Beckman∗ | Roche (E170)† | Siemens∗ | Singulex‡ | |
|---|---|---|---|---|---|
| CV-A (%) | 13.8 | 14.5 | 7.8 | 13.0 | 8.3 |
| CV-I (%) | 15.2 | 6.1 | 15.0 | 12.9 | 9.7 |
| CV-G (%) | 70.5 | 34.8 | NA | 12.3 | 57 |
| Index of individuality | 0.22 | 0.46 | NA | 0.11 | 0.21 |
| RCV (%)§ | NA | NA | 47.0 | NA | NA |
| RCV increase (%)II | 69.3 | 63.8 | NA | 57.5 | 46.0 |
| RCV decrease (%)II | –40.9 | –38.9 | NA | –36.5 | –32 |
| Within-individual mean (ng/L) | 3.5 | 4.9 | NA | 5.5 | 2.8 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download