Abstract
Background
Establishment of a national reference panel for syphilis antibodies is necessary to evaluate the performance of in-vitro diagnostic tests for syphilis and to verify test quality. This study aimed to establish a national reference panel for syphilis antibodies, to assess the suitability of a panel for non-treponemal and treponemal testing, and to assess the reactivity of the various tests currently in use.
Methods
Treponemal pallidum particle agglutination (TPPA)-positive and -negative fresh frozen plasma samples were obtained. After the fresh frozen plasma was converted to serum by defibrination, the samples were pooled. Two candidate reference standards containing no syphilis antibodies and 10 candidate reference standards containing syphilis antibodies were prepared on the basis of reactivity in the TPPA assay. Candidate reference standards were tested by three laboratories using five non-treponemal tests and four treponemal tests.
Results
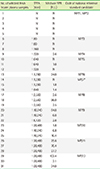
All three laboratories reported positive non-treponemal test results for the mixed-titer performance panel (MP)/6-MP/12. MP/1, MP/2, and MP/3 were negative for non-treponemal tests. MP/4 and MP/5 were reported either as positive or negative according to the laboratories. All laboratories reported positive TPPA results for MP/3-MP/12 and negative results for MP/1 and MP/2. No significant difference was detected among the treponemal testing results in three laboratories.
Conclusions
We established 12 candidate national reference standards containing various concentrations of syphilis antibodies. A collaborative study using nine tests demonstrated that 12 candidate national reference standards presented consistent results, except a few assays with low sensitivity, and thus could be used as a national reference panel for syphilis antibody testing.
Figures and Tables
References
1. Korean Centers for Disease Control and Prevention. Disease web statics system. Updated on Aug 2012. http://www.cdc.go.kr/kcdchome/jsp/observation/stat/submain/subMain.jsp?pageNum=1&sub=3.
2. Choe HS, Lee DS, Lee SJ, Lee CB, Lee WC, Cho YH. Prevalence of sexually transmitted infections and sexual behavior of young adults and middle-aged people presenting to health examination centers in Korea. J Infect Chemother. 2012; 18:207–212.

3. Choe HS, Lee SJ, Kim CS, Cho YH. Prevalence of sexually transmitted infections and the sexual behavior of elderly people presenting to health examination centers in Korea. J Infect Chemother. 2011; 17:456–461.

4. Song EY, Yang JS, Chae SL, Kim S, Choi YS, Cha YJ. Current status of external quality assessment of syphilis test in Korea. Korean J Lab Med. 2008; 28:207–213.

6. Choi KC, Song JY. Recent trends in clinical observation of syphilis and consideration for laboratory tests. J Korean Med Assoc. 2009; 52:1100–1106.

7. Centers for Disease Control and Prevention (CDC). Discordant results from reverse sequence syphilis screening--five laboratories, United States, 2006-2010. MMWR Morb Mortal Wkly Rep. 2011; 60:133–137.
8. French P, Gomberg M, Janier M, Schmidt B, van Voorst Vader P, Young H. IUSTI: 2008 European guidelines on the management of syphilis. Int J STD AIDS. 2009; 20:300–309.

9. World Health Organization. Recommendations for the preparation, characterization and establishment of international and other biological reference standards (revised 2004). Updated on Aug 2012. http://www.who.int/entity/bloodproducts/publications/TRS932Annex2_Inter_biolefstandardsrev2004.pdf.
10. Potkin-Posadskiĭ VA. Defibrination of blood plasma for obtaining hemagglutinating sera. Probl Gematol Pereliv Krovi. 1970; 15:48–49.
11. Castro AR, Kikkert SE, Fears MB, Pope V. Defibrination of blood plasma for use in serological tests for syphilis. Clin Diagn Lab Immunol. 2002; 9:1376–1378.

12. Müller I, Brade V, Hagedorn HJ, Straube E, Schörner C, Frosch M, et al. Is serological testing a reliable tool in laboratory diagnosis of syphilis? Meta-analysis of eight external quality control surveys performed by the german infection serology proficiency testing program. J Clin Microbiol. 2006; 44:1335–1341.

13. Mabey D, Peeling RW, Ballard R, Benzaken AS, Galbán E, Changalucha J, et al. Prospective, multi-centre clinic-based evaluation of four rapid diagnostic tests for syphilis. Sex Transm Infect. 2006; 82:S5. v13–v16.

14. Sato NS, de Melo CS, Zerbini LC, Silveira EP, Fagundes LJ, Ueda M. Assessment of the rapid test based on an immunochromatography technique for detecting anti-Treponema pallidum antibodies. Rev Inst Med Trop Sao Paulo. 2003; 45:319–322.





 PDF
PDF ePub
ePub Citation
Citation Print
Print








 XML Download
XML Download