Abstract
Background
Methods
Results
Conclusions
Figures and Tables
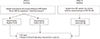 | Fig. 1Schematic diagram and study plan. This study comprised2 phases: in phase I, the laboratory utility of direct measurement of serum immunoglobulin heavy/light chain pairs(the HLC assay) was determined. In phase II, the HLC assay was validated by testing samples showing a definitive monoclonal peak in SPEP and immunotyping.
Abbreviations: SPEP, serum protein electrophoresis; MM, multiple myeloma; HLC assay, heavy chain/light chain assay.
|
 | Fig. 2Linearity results of Hevylite Ig'κ and Ig'λ assays performed using a mixture of normal pooled sera with high concentration controls (simulation test by serial dilution). (A) IgGκ assay, (B) IgGλ assay, (C) IgAκ assay, (D) IgAλ assay. |
 | Fig. 3Serial analysis of sera from an IgGκ multiple myeloma patient; a comparison of serum protein electrophoresis (SPEP), total immunoglobulin G (IgG), monoclonal IgG from SPEP densitometry, and HevyliteTM IgG κ:λ ratio.
Abbreviations: IgG, immunoglobulin G; κ, kappa; λ, lambda; TCD, thalidomide plus cyclophosphamide plus dexamethasone treatment; auto-PBSCT, autologous peripheral blood stem cell transplantation; Vel, velcade (bortezomib); IT, immunotyping; PR, partial response; CR, complete response; NR, normal range.
|
Table 2

*Statistical differences in HLC-IgGκ, HLC-IgGκ/IgGλ ratios, and serum immunotyping results between the suspicious electrophoresis group and the definitive monoclonal peak group were evaluated (P<0.05); †Reference range for the HLC assay: IgGκ, 3.84-12.07 g/L; IgGλ, 1.91-6.74 g/L; IgGκ/IgGλ, 1.12-3.21 g/L; IgAκ, 0.57-2.08 g/L; IgAλ, 0.44-2.04 g/L; IgAκ/IgAλ, 0.78-1.94 g/L.
Abbreviations: Ig, immunoglobulin; sFLC assay, serum free light chain assay; FLCκ, free light chain-kappa; FLCλ, free light chain-lambda; HLC assay, heavy/lightchain assay.
Table 3

*In the case of only numerical data without Ig'κ or FLCκ labeling, this indicates that the result of HLC assay or FLC assay was within the reference range (IgGκ/IgGλ, 1.12-3.21, IgAκ/IgAλ, 0.78-1.94; FLCκ/λ, 0.26-1.65); †Described as per the response criteria given by the International Myeloma Working Group.
Abbreviations: SPEP, serum protein electrophoresis; HLC ratios, heavy/lightchain assay κ:λ ratios; sFLC assay, serum free light chain assay; FLCκ, free light chain-kappa; MM, multiple myeloma; SD, stable disease; PR, partial response; VGPR, very good partial response.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download