Abstract
Background
False negative results have been reported in the immunodetection of hepatitis B virus (HBV) because of the existence of the various mutants of the virus, causing most suppliers to try to develop superior reagents by using highly sensitive and specific monoclonal or polyclonal antibodies. In this study, we evaluated the effectiveness of 3 newly developed reagents by major manufacturers by adopting automated methods with increased sensitivity and specificity in the detection and discrimination of native and recombinant mutant antigens.
Methods
We analyzed samples confirmed positive for hepatitis B surface antigen (HBsAg), high-risk samples from chronic hepatitis patients treated with antiviral agents, and samples from patients who had undergone liver transplantation and were treated with high-dose hepatitis B immunoglobulin (HBIG) by using reagents and systems newly developed by Abbott Laboratories (USA), Roche Diagnostics (Germany), and Siemens Healthcare Diagnostics (USA). Recombinant sample panels from these manufacturers with low and high concentrations were also analyzed for comparing the 3 reagents.
Results
There were no discrepant results among the various selected patient groups; however, for the recombinant mutant panels, all of the 3 reagents showed highly positive detection rates for their corresponding mutant panels, but showed relatively discrepant mutant detection rates when cross-tested with the other mutant panels. Detection rates of the HBsAg mutant panels were higher at a higher concentration of the mutant samples, but were lower for the same mutant receptor sites at a lower concentration.
Conclusions
The 3 major detection methods seem to recognize the major native mutants commonly encountered in clinical practice. However, in the case of recombinant mutants, we believe that our data are not to be interpreted as a reference standard for any reagent, because the results can only be validated for the reagents' corresponding mutant panels; such results tend to be mutually exclusive, and the enough concentration of mutants was required to be adjusted for a comparative analysis.
Figures and Tables
Fig. 1
Correlation of quantitative results between E170 and Architect in Human Serum Bank samples (A), chronic hepatitis B patients with antiviral agents (B) and liver transplanted patient (C).
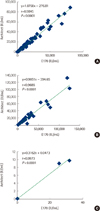
Table 3
Results of immunodetection by using Architect, E170, and Centaur XP for the recombinant mutant panels supplied by Abbott

References
1. World Health Organization. Hepatitis B. World Health Organization Fact Sheet 204 (Revised October 2000). 2000. WHO Web site;http://who.int/inffs/en/fact204.html.
2. Korea Health Statistics 2009 :Korea National Health and Nutrition Examination Survey (KNHANESIV-3).
3. Ganem D, Prince AM. Hepatitis B virus infection--natural history and clinical consequences. N Engl J Med. 2004. 350:1118–1129.

4. Kao JH. Diagnosis of hepatitis B virus infection through serological and virological markers. Expert Rev Gastroenterol Hepatol. 2008. 2:553–562.

5. Leads from the MMWR. Prevention of perinatal transmission of hepatitis B virus: prenatal screening of all pregnant women for hepatitis B surface antigen. JAMA. 1988. 260:165169–170.
6. Weber B. Recent developments in the diagnosis and monitoring of HBV infection and role of the genetic variability of the S gene. Expert Rev Mol Diagn. 2005. 5:75–91.

7. Gerlich WH. Diagnostic problems caused by HBsAg mutants-a consensus report of an expert meeting. Intervirology. 2004. 47:310–313.

8. Coleman PF, Chen YC, Mushahwar IK. Immunoassay detection of hepatitis B surface antigen mutants. J Med Virol. 1999. 59:19–24.

9. Gerner PR, Friedt M, Oettinger R, Lausch E, Wirth S. The hepatitis B virus seroconversion to anti-HBe is frequently associated with HBV genotype changes and selection of preS2-defective particles in chronically infected children. Virology. 1998. 245:163–172.

10. Hsu HY, Chang MH, Ni YH, Lin HH, Wang SM, Chen DS. Surface gene mutants of hepatitis B virus in infants who develop acute or chronic infections despite immunoprophylaxis. Hepatology. 1997. 26:786–791.

11. Lok AS, Zoulim F, Locarnini S, Bartholomeusz A, Ghany MG, Pawlotsky JM, et al. Antiviral drug-resistant HBV: standardization of nomenclature and assays and recommendations for management. Hepatology. 2007. 46:254–265.

12. Ghany MG, Ayola B, Villamil FG, Gish RG, Rojter S, Vierling JM, Lok AS. Hepatitis B virus S mutants in liver transplant recipients who were reinfected despite hepatitis B immune globulin prophylaxis. Hepatology. 1998. 27:213–222.

13. Moerman B, Moons V, Sommer H, Schmitt Y, Stetter M. Evaluation of sensitivity for wild type and mutant forms of hepatitis B surface antigen by four commercial HBsAg assays. Clin Lab. 2004. 50:159–162.
14. Kim H, Jee YM, Song BC, Shin JW, Yang SH, Mun HS, et al. Molecular epidemiology of hepatitis B virus (HBV) genotypes and serotypes in patients with chronic HBV infection in Korea. Intervirology. 2007. 50:52–57.

15. Sumi H, Yokosuka O, Seki N, Arai M, Imazeki F, Kurihara T, et al. Influence of hepatitis B virus genotypes on the progression of chronic type B liver disease. Hepatology. 2003. 37:19–26.

16. Weber B. Genetic variability of the S gene of hepatitis B virus: clinical and diagnostic impact. J Clin Virol. 2005. 32:102–112.

17. Park JW, Yoon JH, Hwang YJ, Lee HS, Kim CY. Mutations at the Gene Encoding the 'a' Determinant of HBsAg in Chronic Hepatitis B Patients with Concurrent HBsAg and Anti-HBs Positivity. Korean J Gastroenterol. 1997. 29:182–191.
18. Hsu HY, Chang MH, Ni YH, Chen HL. Survey of hepatitis B surface variant infection in children 15 years after a nationwide vaccination programme in Taiwan. Gut. 2004. 53:1499–1503.

19. Park NH, Chung WH, Lee HS. Impacts of Vaccination on Hepatitis B Viral Infections in Korea over a 25-Year Period. Intervirology. 2010. 53:20–28.

20. Lee SS, Chang JY, Seo JK. Mutations in Hepatitis B Virus Precore, Core Promoter, and "a" Determinant in Children with Chronic Hepatitis B Virus Infection. Korean J Pediatr Gastroenterol Nutr. 2011. 14:279–285.





 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download