Abstract
Background
Clinical and Laboratory Standards Institute (CLSI) guidelines (H42-A2) recommend the "CD45/SSC" gating method for assays on lymphocyte subset enumeration and CD16 exclusion for assays enumerating NK cells. In contrast, the Flow Cytometry Checklist (06/17/2010) of the College of American Pathology does not recommend a specific lymphocyte gating method, but recommends the correction of lymphocyte subset results for lymphocyte gate purity.
Methods
We compared lymphocyte subset results of EDTA-treated blood from 102 patients with various diseases and 12 normal controls, using 3 lymphocyte gating methods (CD45/SSC, FSC/SSC, and lymphocyte gate purity correction after FSC/SSC gating), and assessed the proportion of CD56-/CD16+ NK cells within the total NK cell population.
Results
Lymphocyte gate purity increased as the percentage of lymphocytes increased. However, lymphocyte subsets that consistently showed high lymphocyte gate purity could not be identified. The purity of the T cell population differed significantly depending on the gating method used: CD45/SSC vs. FSC/SSC, P=0.027; CD45/SSC vs. gate purity correction after FSC/SSC, P=0.002. However, the lymphocyte gate purity correction after FSC/SSC gating did not significantly improve the accuracy of the lymphocyte subset enumeration assay using FSC/SSC gating. The subset of CD56-CD16+ NK cells, constituted an average of 17.1% of total NK cells. Patients had higher proportions of CD56-CD16+ NK cells (13.1-25.5%) than did the normal controls (9.52%).
Figures and Tables
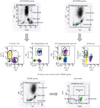 | Fig. 1Assay for enumerating the lymphocyte subset using 3 different gating methods. (A) CD45/SSC gating. (B) FSC/SSC gating. (C) Gate purity correction after FSC/SSC gating. The corrected lymphocyte subset (%) was calculated using the following formulae: *Gate purity (%)=CD45(+) bright/CD14(-) mononuclear cells (%) among lymphocytes by using FSC/SSC gating.
|
 | Fig. 3(A) Correlation between the proportion of lymphocytes and lymphocyte gate purities (r=0.400, P<0.001). (B) Correlation between the proportion of lymphocytes and the differences in T cell values analyzed using CD45/SSC gating and lymphocyte gate purity correction after FSC/SSC gating (r=-0.133, P=0.115). |
 | Fig. 4Distribution of NK cell subsets in patients with various diseases.
*Proportion of NK cells that are CD56-CD16+ NK cells.
Abbreviations: NC, normal control; AML, follow-up of patients with acute myeloid leukemia after chemotherapy; ALL, follow-up of patients with acute lymphoblastic leukemia after chemotherapy; MDS, myelodysplastic syndrome; LC, liver cirrhosis; AA, aplastic anemia; VI, viral infection.
|
Table 1
Percentages of lymphocytic subsets calculated using 3 different lymphocyte gating methods (N=114)
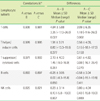
Table 2
Distribution of NK cell subsets according to disease entity
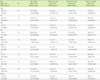
*P<0.05, when data of the NK subset from patient groups were compared with those of the normal controls by using the Mann-Whitney test.
Abbreviations: NC, normal control; VI, viral infection; AML, follow-up of patients with acute myeloid leukemia after chemotherapy; ALL, follow-up of acute lymphoblastic leukemia after chemotherapy; MDS, myelodysplastic syndrome; LC, liver cirrhosis; AA, aplastic anemia.
References
1. Clinical and Laboratory Standards Institute (CLSI). Enumeration of Immunologically Defined Cell Populations by Flow Cytometry, Approved Guideline-Second Edition. CLSI document H42-A2. 2007. Wayne, NJ, USA: CLSI;1821–22.
2. Autissier P, Soulas C, Burdo TH, Williams KC. Evaluation of a 12-Color Flow Cytometry Panel to Study Lymphocyte, Monocyte, and Dendritc Cell Subset in Humans. Cytometry A. 2010. 77:410–419.
3. Siftar Z, Paro MM, Sokolić I, Nazor A, Mestrić ZF. External Quality Assessment in Clinical Cell Analysis by Flow Cytometry. Why is It so Important? Coll Antropol. 2010. 34:207–217.
4. Levering WH, van Wieringen WN, Kraan J, Sintnicolaas K, van Rhenen DJ, Gratama JW. Flow Cytometric Lymphocyte Subset Enumeration: 10 Years of External Quality Assessment in the Benelux Countries. Cytometry B Clin Cytom. 2008. 74:79–90.

5. 2011 College of American Pathologists. 2011 FL-B Flow Cytometry, Participant Summary of SURVEYS 2011.
6. Flow Cytometry Quality Assurance Program supplied by the Korean Society for Laboratory Medicine. 2011-1 Participant Summary of Flow Cytometry Quality Assurance.
7. CAP (College of American Pathologists). Flow Cytometry Checklist (06/17/2010) FLO.30460, FLO.30470.
8. Björkström NK, Ljunggren HG, Sandberg JK. CD56 negative NK cells: origin, function, and role in chronic viral disease. Trends Immunol. 2010. 31(11):401–406.

9. Calvelli T, Denny TN, Paxton H, Gelman R, Kagan J. Guideline for Flow Cytometric Immunophenotyping: a report from the national institute of allergy and infectious diseases, division of AIDS. Cytometry. 1993. 14:702–714.

10. Maria A, Bozzano F, Cantoni C, Moretta L. Revisiting human natural killer cell subset function revealed cytolytic CD56dimCD16+ NK cells as rapid producers of abundant IFN-γ on activation. PNAS. 2011. 108(2):728–732.
11. Gibson SE, Swerdlow SH, Felgar RE. Natural killer cell subsets and natural killer-like T-cell populations in benign and neoplastic B-cell proliferations vary based on clinicopathological features. Human Pathology. 2011. 42:679–687.

12. Feng Y, Zhang R, Zhu H, Peng H, Zhou X, Hong K, Liu J, Chen J, Shao Y. Comparision of the quantities and subset distributions of natural killer cells among different races. Chin Med J. 2010. 123(22):3272–3276.
13. Vieillard V, Fausther-Bovendo H, Samri A, Debré P, et al. Specific Phenotypic and Functional Features of Natural Killer Cells From HIV-Infected Long-Term Nonprogressors and HIV Controllers. J Acquir Immune Defic Syndr. 2010. 53:564–573.

14. Hong HS, Eberhard JM, Keudel P, Bollmann BA, Ahmad F, Ballmaier M, et al. Phenotypically and functionally distinct subsets contribute to the expansion of CD56-/CD16+ natural killer cells in HIV infection. AIDS. 2010. 24:1823–1834.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


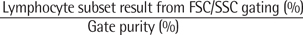

 XML Download
XML Download