Abstract
Background
The HPV28 Detection test (Seegene) is a real-time polymerase chain reaction assay that is designed for testing a total of 28 human papillomavirus (HPV) genotypes and estimating the approximate HPV viral load. The aim of this study was to evaluate the clinical applicability of the HPV28 Detection test with regard to the prevalence of HPV infection and distribution of HPV genotypes by using the HPV28 Detection and HPV DNA Chip tests (Biomedlab).
Methods
HPV DNA Chip and HPV28 Detection tests were performed for 500 cervical swab specimens. HPV genotype results were confirmed by sequencing analysis of the specimens that showed discordant results in the 2 test methods.
Results
The positive rate of HPV detection determined by using HPV28 Detection and HPV DNA Chip tests were 43.8% and 40.6%, respectively. The sequencing results in 64 discordant specimens that showed single HPV infection in the 2 test methods were in complete agreement with the test results obtained with the HPV28 Detection test. The genotyping results of the HPV28 Detection test were 100% concordant in repeated experiments with HPV-infected specimens that have 12 different HPV genotypes, i.e., types 16, 31, 33, 39, 42, 51, 52, 53, 58, 66, 68, and 70. The HPV28 Detection test was 100-fold more sensitive than the HPV DNA Chip test with serially diluted HPV DNAs.
Figures and Tables
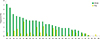 | Fig. 1Frequencies of human papillomavirus genotypes with the HPV28 Detection and HPV DNA Chip tests for 500 cervical specimens. |
Table 1
Positive rate and prevalence of HPV genotypes with the HPV28 Detection and HPV DNA Chip genotyping tests for 500 cervical specimens
References
1. Bosch FX, Lorincz A, Muñoz N, Meijer CJ, Shah KV. The causal relation between human papillomavirus and cervical cancer. J Clin Pathol. 2002; 55:244–265.

2. Sun CA, Liu JF, Wu DM, Nieh S, Yu CP, Chu TY. Viral load of high-risk human papillomavirus in cervical squamous intraepithelial lesions. Int J Gynaecol Obstet. 2002; 76:41–47.

3. Snijders PJ, van den Brule AJ, Meijer CJ. The clinical relevance of human papillomavirus testing: relationship between analytical and clinical sensitivity. J Pathol. 2003; 201:1–6.

4. Kim MK, No JH, Song YS. Human papilloma vaccine. J Korean Med Assoc. 2009; 52:1180–1186.
5. Kim YT, Lee JM, Hur SY, Cho CH, Kim YT, Kim SC, et al. Clearance of human papillomavirus infection after successful conization in patients with cervical intraepithelial neoplasia. Int J Cancer. 2010; 126:1903–1909.

6. Lee MY, Cho CH, Kwon SH, Song DK, Chung SW, Kang HO, et al. Annual report of gynecologic cancer registry program in Korean for 2002 (Jan. 1st, 2002-Dec. 31st, 2002). Korean J Obstet Gynecol. 2004; 47:S6. 1029–1070.
7. Schiffman M, Adrianza ME. ASCUS-LSIL Triage Study. Design, methods and characteristics of trial participants. Acta Cytol. 2000; 44:726–742.
8. Koss LG. The Papanicolaou test for cervical cancer detection. A triumph and a tragedy. JAMA. 1989; 261:737–743.

9. Miller AB, Nazeer S, Fonn S, Brandup-Lukanow A, Rehman R, Cronje H, et al. Report on consensus conference on cervical cancer screening and management. Int J Cancer. 2000; 86:440–447.

10. Monsonego J, Bosch FX, Coursaget P, Cox JT, Franco E, Frazer I, et al. Cervical cancer control, priorities and new directions. Int J Cancer. 2004; 108:329–333.

11. Nanda K, McCrory DC, Myers ER, Bastian LA, Hasselblad V, Hickey JD, et al. Accuracy of the Papanicolaou test in screening for and follow-up of cervical cytologic abnormalities; a systematic review. Ann Intern Med. 2000; 132:810–819.

12. Fahey MT, Irwig L, Macaskill P. Meta-analysis of Pap test accuracy. Am J Epidemiol. 1995; 141:680–686.

13. Muñoz N, Bosch FX, de Sanjosé S, Herrero R, Castellsagué X, Shah KV, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003; 348:518–527.

14. Lee EH, Lee KB, Kim EO, Ji SI, Shin SK, Lee HS, et al. Clinical effectiveness of restriction fragment mass polymorphism assay for human papillomavirus genotyping. J Lab Med Qual Assur. 2008; 30:291–299.
15. Bosch FX, Manos MM, Muñoz N, Sherman M, Jansen AM, Peto J, et al. International biological study on cervical cancer (IBSCC) Study Group. Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. J Natl Cancer Inst. 1995; 87:796–802.

16. Castellsagué X, Díaz M, de Sanjosé S, Muñoz N, Herrero R, Franceschi S, et al. Worldwide human papillomavirus etiology of cervical adenocarcinoma and its cofactors: implications for screening and prevention. J Natl Cancer Inst. 2006; 98:303–315.

17. Clifford GM, Smith JS, Plummer M, Muñoz N, Franceschi S. Human papillomavirus types in invasive cervical cancer worldwide: a meta-analysis. Br J Cancer. 2003; 88:63–73.

18. Hwang HS, Park M, Lee SY, Kwon KH, Pang MG. Distribution and prevalence of human papillomavirus genotypes in routine pap smear of 2,470 Korean women determined by DNA chip. Cancer Epidemiol Biomarkers Prev. 2004; 13:2153–2156.
19. An HJ, Cho NH, Lee SY, Kim IH, Lee C, Kim SJ, et al. Correlation of cervical carcinoma and precancerous lesions with human papillomavirus (HPV) genotypes detected with the HPV DNA chip microarray method. Cancer. 2003; 97:1672–1680.

20. Cho NH, An HJ, Jeong JK, Kang S, Kim JW, Kim YT, et al. Genotyping of 22 human papillomavirus types by DNA chip in Korean women: comparison with cytologic diagnosis. Am J Obstet Gynecol. 2003; 188:56–62.

21. Hong SR, Kim IS, Kim DW, Kim MJ, Kim AR, Kim YO, et al. Prevalence and genotype distribution of cervical human papillomavirus DNA in Korean women: a multicenter study. Korean J Pathol. 2009; 43:342–350.

22. Bae JH, Lee SJ, Kim CJ, Hur SY, Park YG, Lee WC, et al. Human papillomavirus (HPV) type distribution in Korean women: a meta-analysis. J Microbiol Biotechnol. 2008; 18:788–794.
23. Shin HR, Franceschi S, Vaccarella S, Roh JW, Ju YH, Oh JK, et al. Prevalence and determinants of genital infection with papillomavirus, in female and male university students in Busan, South Korea. J Infect Dis. 2004; 190:468–476.

24. Oh JK, Franceschi S, Kim BK, Kim JY, Ju YH, Hong EK, et al. Prevalence of human papillomavirus and Chlamydia trachomatis infection among women attending cervical cancer screening in the Republic of Korea. Eur J Cancer Prev. 2009; 18:56–61.

25. Kim YJ, Kwon MJ, Woo HY, Paik SY. Prevalence of Human Papillomavirus Infection and Genotype Distribution Determined by the Cyclic-Catcher Melting Temperature Analysis in Korean medical checkup population. J Microbiol. 2013; (in press).

26. Fuessel Haws AL, He Q, Rady PL, Zhang L, Grady J, Hughes TK, et al. Nested PCR with the PGMY09/11 and GP5(+)/6(+) primer sets improves detection of HPV DNA in cervical samples. J Virol Methods. 2004; 122:87–93.

27. Ylitalo N, Sørensen P, Josefsson AM, Magnusson PK, Andersen PK, Pontén J, et al. Consistent high viral load of human papillomavirus 16 and risk of cervical carcinoma in situ: a nested case-control study. Lancet. 2000; 355:2194–2198.

28. Elfgren K, Kalantari M, Moberger B, Hagmar B, Dillner J. A population-based five-year follow-up study of cervical human papillomavirus infection. Am J Obstet Gynecol. 2000; 183:561–567.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download