Abstract
Background
Magicplex HepaTrio Real-time PCR (Magicplex, Seegene, Korea) simultaneously detects and distinguishes each type of hepatitis A, hepatitis B, and hepatitis C viruses. We investigated the diagnostic performance of Magicplex in comparison with that of serologic test.
Methods
We tested and analyzed 184 serum samples for hepatitis A IgM antibody (IgM antiHAV), hepatitis B virus surface antigen (HBsAg), and anti-hepatitis C virus antibody (antiHCV). Serologic markers including IgM antiHAV, HBsAg, and antiHCV were tested with electrochemiluminescence immunoassay method. We calculated positive rates of the test results and concordance rates between serologic tests and Magicplex.
Results
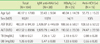
The positive rates of IgM antiHAV, HBsAg, and antiHCV using serologic methods were 15.2% (28/184), 13.6% (25/184), and 8.2% (15/184), respectively. The positive rates of the corresponding viral nucleic acid detection by Magicplex were 18.5% (34/184), 16.3% (30/184), and 4.3% (8/184), respectively. The concordance rates between serologic test and Magicplex were 95.7% (176/184) in hepatitis A, 97.3% (179/ 184) in hepatitis B, and 96.2% (177/184) in hepatitis C.
Conclusions
In our study, the concordance rates between Magicplex and traditional serologic tests are over 95%. Magicplex could not yet totally replace traditional serologic tests because there are some possibilities of cross reaction among the hepatitis viruses and false negative results in hepatitis C. If Magicplex resolves these problems, it would be a useful tool for screening test for the diagnosis of viral hepatitis as it provides an automated, easy, and simultaneous detection of the 3 major hepatitis viruses.
Figures and Tables
References
1. Kang HM, Jeong SH, Kim JW, Lee D, Choi CK, Park YS, et al. Recent etiology and clinical features of acute viral hepatitis in a single center of Korea. Korean J Hepatol. 2007. 13:495–502.

2. Lee DH, Kim JH, Nam JJ, Kim HR, Shin HR. Epidemiological findings of hepatitis B infection based on 1998 National Health and Nutrition Survey in Korea. J Korean Med Sci. 2002. 17:457–462.

3. Pawlotsky JM. Therapy of hepatitis C: from empiricism to eradication. Hepatology. 2006. 43(Suppl 1):S207–S220.

4. Shin HR, Kim JY, Kim JI, Lee DH, Yoo KY, Lee DS, et al. Hepatitis B and C virus prevalence in a rural area of South Korea: the role of acupuncture. Br J Cancer. 2002. 87:314–318.

5. Shin HR, Kim JY, Ohno T, Cao K, Mizokami M, Risch H, et al. Prevalence and risk factors of hepatitis C virus infection among Koreans in rural area of Korea. Hepatol Res. 2000. 17:185–196.

6. Huh HJ, Chae SL, Cha YJ. Comparison study with enzyme immunoassay and chemiluminescence immunoassay for hepatitis B virus surface antigen detection. Korean J Lab Med. 2007. 27:355–359.

7. Kee SJ, Shin JH, Suh SP, Ryang DW. Discrepancy between serological markers of enzyme immunoassay and results of polymerase chain reaction for hepatitis B and C viruses in patients with chronic liver diseases. Korean J Clin Pathol. 1996. 16:965–978.
8. Hsu HH, Gonzalez M, Foung SK, Feinstone SM, Greenberg HB. Antibodies to hepatitis C virus in lowrisk blood donors: implications for counseling positive donors. Gastroenterology. 1991. 101:1724–1727.

9. Sugitani M, Inchauspé G, Shindo M, Prince AM. Sensitivity of serological assays to identify blood donors with hepatitis C viraemia. Lancet. 1992. 339:1018–1019.

10. Pramoolsinsap C. Acute hepatitis A and acquired immunity to hepatitis A virus in hepatitis B virus (HBV) carriers and in HBVor hepatitis C virusrelated chronic liver diseases in Thailand. J Viral Hepat. 2000. 7:Suppl 1. 11–12.

11. Chu CM, Liaw YF. Increased incidence of fulminant hepatic failure in previously unrecognized HBsAg carriers with acute hepatitis independent of etiology. Infection. 2005. 33:136–139.

12. Kim YK, Kim BH, Jin ES, Nam KD, Jang JY, Kim NH, et al. Positive predictability and predictive factors of the third generation antihepatitis C virus (HCV) ELISA test for HCV infection. Korean J Gastroenterol. 2005. 45:181–188.
13. Liaw YF. Role of hepatitis C virus in dual and triple hepatitis virus infection. Hepatology. 1995. 22:1101–1108.

14. Buxton JA, Kim JH. Hepatitis A and hepatitis B vaccination responses in persons with chronic hepatitis C infections: A review of the evidence and current recommendations. Can J Infect Dis Med Microbiol. 2008. 19:197–202.

15. Raimondo G, Cacciamo G, Saitta C. Hepatitis B virus and hepatitis C virus coinfection: additive players in chronic liver disease. Ann Hepatol. 2005. 4:100–106.

16. Lee EJ, Kwon SY, Seo TH, Yun HS, Cho HS, Kim BK, et al. Clinical features of acute hepatitis A in recent two years. Korean J Gastroenterol. 2008. 52:298–303.
17. Hirata R, Hoshino Y, Sakai H, Marumo F, Sato C. Patients with hepatitis A with negative IgMHA antibody at early stages. Am J Gastroenterol. 1995. 90:1168–1169.
18. Weber B. Genetic variability of the S gene of hepatitis B virus: clinical and diagnostic impact. J Clin Virol. 2005. 32:102–112.

19. Koyanagi T, Nakamuta M, Sakai H, Sugimoto R, Enjoji M, Koto K, et al. Analysis of HBs antigen negative variant of hepatitis B virus: unique substitutions, Glu129 to Asp and Gly145 to Ala in the surface antigen gene. Med Sci Monit. 2000. 6:1165–1169.
20. Jilg W, Sieger E, Zachoval R, Schätzl H. Individuals with antibodies against hepatitis B core antigen as the only serological marker for hepatitis B infection: high percentage of carriers of hepatitis B and C virus. J Hepatol. 1995. 23:14–20.

21. Coleman PF, Chen YC, Mushahwar IK. Immunoassay detection of hepatitis B surface antigen mutants. J Med Virol. 1999. 59:19–24.

22. Mosley JW, Aach RD, Hollinger FB, Stevens CE, Barbosa LH, Nemo GJ, et al. NonA, nonB hepatitis and antibody to hepatitis C virus. JAMA. 1990. 263:77–78.

23. Hur M, Kang HJ, Kang SH, Lee KM. Evaluation of ADVIA Centaur HCV Assay for the Detection of Hepatitis C Virus Antibody: A Comparison Study with AxSYM HCV Version 3.0 Assay. Korean J Lab Med. 2005. 25:181–185.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download