1. Lee WM. Hepatitis B virus infection. N Engl J Med. 1997; 337:1733–1745.

2. Lee HS. Current status of viral hepatitis and serological diagnosis. Korean J Clin Pathol. 1995; 15(S2):S197–S212.
3. Lee DH, Kim JH, Nam JJ, Kim HR, Shin HR. Epidemiological findings of hepatitis B infection based on 1998 National Health and Nutrition Survey in Korea. J Korean Med Sci. 2002; 17:457–462.

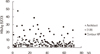
4. Su TH, Hsu CS, Chen CL, Liu CH, Huang YW, Tseng TC, et al. Serum hepatitis B surface antigen concentration correlates with HBV DNA level in patients with chronic hepatitis B. Antivir Ther. 2010; 15:1133–1139.

5. Wursthorn K, Lutgehetmann M, Dandri M, Volz T, Buggisch P, Zollner B, et al. Peginterferon alpha-2b plus adefovir induce strong cccDNA decline and HBsAg reduction in patients with chronic hepatitis B. Hepatology. 2006; 44:675–684.

6. Chen CH, Lee CM, Wang JH, Tung HD, Hung CH, Lu SN. Correlation of quantitative assay of hepatitis B surface antigen and HBV DNA levels in asymptomatic hepatitis B virus carriers. Eur J Gastroenterol Hepatol. 2004; 16:1213–1218.

7. Jilg W, Sieger E, Zachoval R, Schatzl H. Individuals with antibodies against hepatitis B core antigen as the only serological marker for hepatitis B infection: high percentage of carriers of hepatitis B and C virus. Journal of Hepatology. 1995; 23:14–20.

8. Coleman PF, Chen YC, Mushahwar IK. Immunoassay detection of hepatitis B surface antigen mutants. J Med Virol. 1999; 59:19–24.

9. Weber B, Dengler T, Berger A, Doerr HW, Rabenau H. Evaluation of two new automated assays for hepatitis B virus surface antigen (HBsAg) detection: IMMULITE HBsAg and IMMULITE 2000 HBsAg. J Clin Microbiol. 2003; 41:135–143.

10. Moerman B, Moons V, Sommer H, Schmitt Y, Stetter M. Evaluation of sensitivity for wild type and mutant forms of hepatitis B surface antigen by four commercial HBsAg assays. Clin Lab. 2004; 50:159–162.
11. Weber B. Genetic variability of the S gene of hepatitis B virus: clinical and diagnostic impact. J Clin Virol. 2005; 32:102–112.

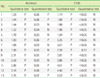
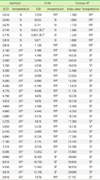
12. Song SM, Oh WI, Kim DW. Evaluation of Serologic Marker Tests for Hepatitis B Viral Infection Using the Automated Immunoassay System ARCHITECT i2000. Korean J Clin Pathol. 2002; 22:42–46.
13. Yoo SJ, Oh HJ, Shin BM. Comparison of 3 Automated Immunoassay for Hepatitis B surface Antigen. Korean J Lab Med. 2006; 26:282–286.

14. Cha YJ, Kwon SY, Kim TY, Kim JR, Kim HS, Park MH, et al. Annual report on external quality assessment in immunoserology in Korea(2005). J Lab Med Qual Assur. 2006; 28:41–61.
15. Cha YJ, Kwon SY, Kim TY, Kim JR, Kim HS, Park MH, et al. Annual report on external quality assessment in immunoserology in Korea(2006). J Lab Med Qual Assur. 2007; 29:45–64.
16. Cha YJ, Kwon SY, Kim TY, Kim JR, Kim HS, Park MH, et al. Annual report on external quality assessment in immunoserology in Korea(2007). J Lab Med Qual Assur. 2008; 30:49–74.
17. Cha YJ, Kwon SY, Kim TY, Kim JR, Kim HS, Park MH, et al. Annual report on external quality assessment in immunoserology in Korea(2008). J Lab Med Qual Assur. 2009; 31:49–72.
18. Chen D, Kaplan L, Liu Q. Evaluation of two chemiluminescent immunoassays of ADVIA Centaur for hepatitis B serology markers. Clin Chim Acta. 2005; 355:41–45.

19. Taylor P, Pickard G, Gammie A, Atkins M. Comparison of the ADVIA Centaur and Abbott AxSYM immunoassay systems for a routine diagnostic virology laboratory. J Clin Virol. 2004; 30:Suppl 1. S11–S15.







 PDF
PDF ePub
ePub Citation
Citation Print
Print








 XML Download
XML Download