Abstract
In Korea, the majority of imported malaria cases are Plasmodium vivax and P. falciparum, but Plasmodium ovale cases are rarely reported. We describe an imported case of P. ovale that was confirmed by peripheral blood smear and nested PCR targeting the small subunit ribosomal RNA (SSU rRNA) gene. A 37-yr-old male had visited the Republic of Ghana in tropical West Africa 3 months ago, and suffered from fever and headache since 2 weeks after his return to Korea. The results of rapid malaria test using SD Malaria Antigen/Antibody Kit (Standard Diagnostics, Korea) were negative, but Plasmodium species was observed in Wright-Giemsa-stained peripheral blood smear. For the evaluation of possible mixed infection and identification of species, we performed a nested PCR targeting the SSU rRNA gene. P. ovale single infection was confirmed by PCR. The sequence analysis of the P. ovale SSU rRNA gene showed that our isolate was P. ovale classic type. We should confirm P. ovale infection for an accurate diagnosis and treatment of imported malaria cases in Korea because the number of travelers to P. ovale-endemic regions has recently increased.
Figures and Tables
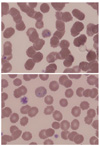 | Fig. 1Wright-Giemsa-stained peripheral blood smear (magnification, ×1,000). A ring form of malaria trophozoite in an enlarged red blood cell with fimbriated margin is observed in each of the two pictures. |
 | Fig. 2
Plasmodium species-specific nested PCR. The Plasmodium genus can be identified from the presence of first-round amplification product (1,000 bp). Plasmodium species can be identified from the presence of second-round amplification products specific for P. vivax (144 bp), P. falciparum (205 bp), and P. ovale (788 bp) respectively. Only P. ovale DNA was detected from the patient's blood. (P, positive control; N, negative control; Pt., Patient's peripheral blood sample; M, DNA ladder marker). |
 | Fig. 3Phylogenetic tree based on the small subunit ribosomal RNA genes of Plasmodium species including eight registered P. ovale isolates (Nigerian I/CDC, Papua New Guinea, MAL/MAI, CAG/Cameroon, classic types, and variant types) and P. ovale isolate (SM10-201) of this study. SM10-201 is more similar to the isolates of P. ovale classic type1 than other isolates. |
References
2. Korea Centers for Disease Control and Prevention. 2009 Guidelines of malaria. 2009. Seoul, Korea: The Korea Centers for Disease Control and Prevention;47.
3. Han TH, Kim BN, Seong HK. A case of imported Plasmodium ovale malaria. J Korean Med Sci. 2006. 21:932–935.
4. Snounou G, Viriyakosol S, Zhu XP, Jarra W, Pinheiro L, do Rosario VE, et al. High sensitivity of detection of human malaria parasites by the use of nested polymerase chain reaction. Mol Biochem Parasitol. 1993. 61:315–320.

5. Kumar S, Tamura K, Nei M. MEGA3: Integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief Bioinform. 2004. 5:150–163.

6. Toma H, Kobayashi J, Vannachone B, Arakawa T, Sato Y, Nambanya S, et al. Plasmodium ovale infections detected by PCR assay in Lao PDR. Southeast Asian J Trop Med Public Health. 1999. 30:620–622.
7. Win TT, Lin K, Mizuno S, Zhou M, Liu Q, Ferreira MU, et al. Wide distribution of Plasmodium ovale in Myanmar. Trop Med Int Health. 2002. 7:231–239.
8. Kawamoto F, Liu Q, Ferreira MU, Tantular IS. How prevalent are Plasmodium ovale and P. malariae in East Asia? Parasitol Today. 1999. 15:422–426.
10. Park JW, Jun G, Yeom JS. Plasmodium vivax malaria: status in the Republic of Korea following reemergence. Korean J Parasitol. 2009. 47:Suppl. S39–S50.
11. Park TS, Kim JH, Kang CI, Lee BH, Jeon BR, Lee SM, et al. Diagnostic usefulness of SD malaria antigen and antibody kits for differential diagnosis of vivax malaria in patients with fever of unknown origin. Korean J Lab Med. 2006. 26:241–245.

12. May J, Mockenhaupt FP, Ademowo OG, Falusi AG, Olumese PE, Bienzle U, et al. High rate of mixed and subpatent malarial infections in southwest Nigeria. Am J Trop Med Hyg. 1999. 61:339–343.

13. Tangpukdee N, Thanachartwet V, Krudsood S, Luplertlop N, Pornpininworakij K, Chalermrut K, et al. Minor liver profile dysfunctions in Plasmodium vivax, P. malaria and P. ovale patients and normalization after treatment. Korean J Parasitol. 2006. 44:295–302.

14. Song HS, Kim JM, Na DJ, Kim JY, Kim YJ, Yeom JS, et al. The first case of plasmodium malariae infection imported from Nigeria to Korea. Korean J Med. 2009. 77:Suppl 5. S1323–S1327.
15. Mueller I, Zimmerman PA, Reeder JC. Plasmodium malariae and plasmodium ovale-the "bashful" malaria parasites. Trends Parasitol. 2007. 23:278–283.
16. Win TT, Jalloh A, Tantular IS, Tsuboi T, Ferreira MU, Kimura M, et al. Molecular analysis of Plasmodium ovalevariants. Emerg Infect Dis. 2004. 10:1235–1240.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download