Abstract
Background
Because of the long time required for conventional drug susceptibility test (DST) for rifampin and isoniazid, development of rapid DSTs is necessary. Recently, the AdvanSure™ MDR-TB GenoBlot Assay kit (LG Life Science, Korea), using reverse hybridization line blot assay, was developed. We compared this kit with Genotype® MTBDRplus (HAIN Lifescience, Germany) and conventional DST.
Methods
Of the DNAs preserved after performing DST by using Genotype®, we selected 144 samples having conventional DST results. The experiments with both the kits were performed according to the manufacturers' instructions. For the samples for which discrepant results were obtained, sequencing was performed if the DNA was available. Conventional DST was performed at the Korean Institute of Tuberculosis by using the absolute concentration method.
Results
For rifampin, the findings obtained using both the kits were the same with concordance rates of 98.6% (142/144) compared to conventional DST. Of the 2 discrepant findings, one was very major error and the other was major error. For isoniazid, compared to conventional DST, concordance rates of AdvanSure™ and Genotype® were 95.8%(138/144) and 95.1%(137/144) respectively. Of the 6 discrepant findings between conventional method and Advansure™, 5 were very major error and one was major error. All the 7 discrepant findings between conventional method and Genotype® were very major error.
Figures and Tables
Table 1
Mutations in the rpoB, katG, inhA, and ahpC genes and the corresponding wild-type and mutation probes in the AdvanSure™ MDR-TB GenoBlot Assay kit
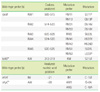
Table 2
Mutations in the rpoB, katG, and inhA genes and the corresponding wild-type and mutation probes in the Genotype® MTBDRplus kit
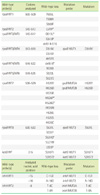
Table 5
The number of mutations associated with RIF and INH resistance detected by AdvanSure™ MDR-TB GenoBlot Assay

References
1. Centers for Disease Control and Prevention (CDC). Emergence of Mycobacterium tuberculosis with extensive resistance to second-line drugs -- worldwide, 2000-2004. MMWR Morb Mortal Wkly Rep. 2006. 55:301–305.
2. Bai GH, Park YK, Choi YW, Bai JI, Kim HJ, Chang CL, et al. Trend of anti-tuberculosis drug resistance in Korea, 1994-2004. Int J Tuberc Lung Dis. 2007. 11:571–576.
3. Kim BJ, Lee IH, Lee DH, Bai GH, Kong SJ, Lee SH, et al. The current status of multidrug-resistant tuberculosis in Korea. Tuberc Respir Dis. 2006. 60:404–411.

4. Rossau R, Traore H, De Beenhouwer H, Mijs W, Jannes G, De Rijk P, et al. Evaluation of the INNO-LiPA Rif. TB assay, a reverse hybridization assay for the simultaneous detection of Mycobacterium tuberculosis complex and its resistance to rifampin. Antimicrob Agents Chemother. 1997. 41:2093–2098.

5. Shin DH. New diagnostic methods for Mycobacterium tuberculosis infection. J Korean Med Assoc. 2006. 49:773–780.

6. Murray PR, Baron EJ, editors. Manual of clinical microbiology. 2007. 9th ed. Washington DC: American Society for Microbiology;1255.
7. Huyen MN, Tiemersma EW, Lan NT, Cobelens FG, Dung NH, Sy DN, et al. Validation of the GenoType MTBDRplus assay for diagnosis of multidrug resistant tuberculosis in South Vietnam. BMC Infect Dis. 2010. 10:149.

8. Huang WL, Chen HY, Kuo YM, Jou R. Performance assessment of the GenoType MTBDRplus test and DNA sequencing in detection of multidrug-resistant Mycobacterium tuberculosis. J Clin Microbiol. 2009. 47:2520–2524.

9. Guo Y, Zhou Y, Wang C, Zhu L, Wang S, Li Q, et al. Rapid, accurate determination of multidrug resistance in M. tuberculosis isolates and sputum using a biochip system. Int J Tuberc Lung Dis. 2009. 13:914–920.
10. Sandgren A, Strong M, Muthukrishnan P, Weiner BK, Church GM, Murray MB. Tuberculosis drug resistance mutation database. PloS Med. 2009. 6:e2.

11. Tuberculosis drug resistance mutation database. Updated on April 2010. http://www.tbdreamdb.com/INH.html.
12. Doustdar F, Khosravi AD, Farnia P, Masjedi MR, Velayati AA. Molecular analysis of isoniazid resistance in different genotypes of Mycobacterium tuberculosis isolates from Iran. Microb Drug Resist. 2008. 14:273–279.

13. Hofmann-Thiel S, van Ingen J, Feldmann K, Turaev L, Uzakova GT, Murmusaeva G, et al. Mechanisms of heteroresistance to isoniazid and rifampin of Mycobacterium tuberculosis in Tashkent, Uzbekistan. Eur Respir J. 2009. 33:368–374.

14. Rinder H, Mieskes KT, Löscher T. Heteroresistance in Mycobacterium tuberculosis. Int J Tuberc Lung Dis. 2001. 5:339–345.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download