Abstract
Background
Leukoreduced blood components are recommended for prevention of non-hemolytic febrile transfusion reactions, HLA alloimmunization, platelet transfusion refractoriness, and transfusion-transmissible diseases. In addition, prestorage leukoreduction may be advantageous to poststorage leukoreduction. The authors investigated the current status of usage of leukoreduced blood components in Korea.
Methods
We surveyed 2,373 medical facilities, where blood components were supplied from Korean Red Cross blood centers and/or Hanmaeum blood center during one year period between January and December 2009. The survey was conducted about the current situation of usage of leukoreduction by web-based program (http://bms.cdc.go.kr), and 743 facilities answered and were analyzed.
Results
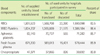
The leukoreduced RBC components comprised 10.3% (prestorage leukoreduction, 91,066 units, 5.7%; poststorage leukoreduction 73,192 units, 4.6%) of the total 1,593,098 units of RBC components used in 743 medical facilities. The leukoreduced platelet concentrates comprised 33.1% (458,552 units) of the total 1,386,184 units of platelet concentrates used in 397 medical facilities. If 1 single donor platelet is counted as 6 platelet concentrates, 48.9% of the total platelet components used were leukoreduced.
Conclusions
The proportion of leukoreduced blood components to the total blood components used in Korea was much lower than that in Unites States of America, especially lower in the use of prestorage leukoreduction of RBC components. Further studies are required for cost-effectiveness and demand-supply amounts of leukoreduced blood components, and appropriate prestorage leukoreduction has to be performed in Korea based on these studies.
Figures and Tables
References
1. Lane TA. Leukocyte reduction of cellular blood components. Effectiveness, benefits, quality control, and costs. Arch Pathol Lab Med. 1994. 118:392–404.
2. Miller JP, Mintz PD. The use of leukocyte-reduced blood components. Hematol Oncol Clin North Am. 1995. 9:69–90.

3. Dzik S, Aubuchon J, Jeffries L, Kleinman S, Manno C, Murphy MF, et al. Leukocyte reduction of blood components: public policy and new technology. Transfus Med Rev. 2000. 14:34–52.

4. Ratko TA, Cummings JP, Oberman HA, Crookston KP, DeChristopher PJ, Eastlund DT, et al. Evidence-based recommendations for the use of WBC-reduced cellular blood components. Transfusion. 2001. 41:1310–1319.

5. Da Ponte A, Bidoli E, Talamini R, Steffan A, Abbruzzese L, Toffola RT, et al. Pre-storage leucocyte depletion and transfusion reaction rates in cancer patients. Transfus Med. 2005. 15:37–43.

6. Hammer JH, Mynster T, Reimert CM, Pedersen AN, Nielsen HJ. Reduction of bioactive substances in stored donor blood: prestorage versus bedside leucofiltration. Eur J Haematol. 1999. 63:29–34.

7. Sweeney JD. Universal leukoreduction of cellular blood components in 2001? Yes. Am J Clin Pathol. 2001. 115:666–673.

8. Heddle NM, Klama L, Meyer R, Walker I, Boshkov L, Roberts R, et al. A randomized controlled trial comparing plasma removal with white cell reduction to prevent reactions to platelets. Transfusion. 1999. 39:231–238.

9. Ledent E, Berlin G. Inadequate white cell reduction by bedside filtration of red cell concentrates. Transfusion. 1994. 34:765–768.

10. Popovsky MA. Quality of blood components filtered before storage and at the beside: implications for transfusion practice. Transfusion. 1996. 36:470–474.

11. Bassuni WY, Blajchman MA, Al-Moshary MA. Why implement universal leukoreduction? Hematol Oncol Stem Cell Ther. 2008. 1:106–123.

12. Report of the US Department of Health and Human Services. The 2009 national blood collection and utilization survey report. 2011. Washington, DC: US Department of Health and Human Services, Office of the Assistant Secretary for Health.
13. Kim S. Development of national blood collection and utilization data monitoring system. 2010. Seoul: National Institute of Health, Korea.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download