Abstract
Background
Urine ketone test is commonly used to screen for diabetic ketoacidosis (DKA). Ketonuria also develops in patients with disease conditions other than DKA. However, the prevalence of DKA in patients with ketonuria is not known. We investigated the prevalence of ketonuria and characteristics of patients with ketonuria and estimated the prevalence of DKA among them to study the clinical significance of ketonuria as an indicator of DKA.
Methods
We studied 1,314 adult and 1,027 pediatric patients who underwent urinalysis. The prevalence of ketonuria in the different groups of patients, classified according to the types of their visits to the institution, was investigated, and the relationships between ketonuria and albuminuria, glycosuria, and bilirubinuria were evaluated.
Results
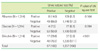
The overall prevalence of ketonuria was 9.1%. The prevalences of ketonuria in adult and pediatric patients were 4.3% and 15.2%, respectively. The prevalences of ketonuria were the highest in the adult (9.7%) and pediatric (28%) patients in the group that had visited the emergency department. Among patients with ketonuria, 7% adult and 3.8% pediatric patients showed glycosuria.
Conclusions
This study showed that the prevalence of DKA in patients with ketonuria, defined as the simultaneous presence of ketone bodies and glucose in urine, was only 7%. Therefore, we concluded that ketonuria might be clinically significant as an indicator of acute or severe disease status rather than of DKA.
Figures and Tables
Table 1
Ketonuria in adult patients classified according to the types of their visits to the hospital

Table 2
Ketonuria in pediatric patients classified according to the types of their visit to the hospital

References
1. Laffel L. Ketone bodies: a review of physiology, pathophysiology and application of monitoring to diabetes. Diabetes Metab Res Rev. 1999. 15:412–426.

2. McGarry JD, Foster DW. Regulation of hepatic fatty acid oxidation and ketone body production. Annu Rev Biochem. 1980. 49:395–420.

3. Hendey GW, Schwab T, Soliz T. Urine ketone dip test as a screen for ketonemia in diabetic ketoacidosis and ketosis in the emergency department. Ann Emerg Med. 1997. 29:735–738.

4. Schwab TM, Hendey GW, Soliz TC. Screening for ketonemia in patients with diabetes. Ann Emerg Med. 1999. 34:342–346.

5. Platia EV, Hsu TH. Hypoglycemic coma with ketoacidosis in nondiabetic alcoholics. West J Med. 1979. 131:270–276.
6. Ngatchu T, Sangwaiya A, Dabiri A, Dhar A, McNeil I, Arnold JD. Alcoholic ketoacidosis with multiple complications: a case report. Emerg Med J. 2007. 24:776–777.

8. Kabadi UM. Pancreatic ketoacidosis: ketonemia associated with acute pancreatitis. Postgrad Med J. 1995. 71:32–35.

9. Bock DE, Rupar CA, Prasad C. Asymptomatic critical hypoglycemia: a dangerous presentation of glycogen storage disease type Ib in infancy. Acta Paediatr. 2011. 100:e130–e132.
10. Rewers M, Pihoker C, Donaghue K, Hanas R, Swift P, Klingensmith GJ. Assessment and monitoring of glycemic control in children and adolescents with diabetes. Pediatric Diabetes. 2007. 8:408–418.

11. Weinstein DA, Correia CE, Saunders AC, Wolfsdorf JI. Hepatic glycogen synthase deficiency: an infrequently recognized cause of ketotic hypoglycemia. Mol Genet Metab. 2006. 87:284–288.

12. Mangione F, Calcaterra V, Esposito C, Dal Canton A. Renal blood flow redistribution during acute kidney injury. Am J Kidney Dis. 2010. 56:785–787.

14. Chalmers RJ, Sulaiman WR, Johnson RH. The metabolic response to exercise in chronic alcoholics. Q J Exp Physiol Cogn Med Sci. 1977. 62:265–274.

16. Féry F, Balasse EO. Ketone body production and disposal in diabetic ketosis. A comparison with fasting ketosis. Diabetes. 1985. 34:326–332.

17. Patel A, Felstead D, Doraiswami M, Stocks GM, Waheed U. Acute starvation in pregnancy: a cause of severe metabolic acidosis. Int J Obstet Anesth. 2011. 20:253–256.

18. Reichard GA Jr, Owen OE, Haff AC, Paul P, Bortz WM. Ketone-body production and oxidation in fasting obese humans. J Clin Invest. 1974. 53:508–515.

19. Minnitt RJ. A successful treatment for toxic symptoms resulting from ether anaesthesia, based on a biochemical investigation. Proc R Soc Med. 1933. 26:347–355.

20. Lee JI, Sohn TS, Chang SA, Lee JM, Cha BY, Son HY, et al. Clinical characteristics and outcomes of diabetic ketoacidosis at a single institution. Korean Diabetes J. 2008. 32:165–170.

21. Taboulet P, Haas L, Porcher R, Manamani J, Fontaine JP, Feugeas JP, et al. Urinary acetoacetate or capillary beta-hydroxybutyrate for the diagnosis of ketoacidosis in the emergency department setting. Eur J Emerg Med. 2004. 11:251–258.

22. Joo NS, Lee DJ, Kim KM, Kim BT, Kim CW, Kim KN. Ketonuria after fasting may be related to the metabolic superiority. J Korean Med Sci. 2010. 25:1771–1776.

23. Kitabchi AE, Fisher JN. Glew RA, Peters SP, editors. Diabetes mellitus. Clinical studies in medical biochemistry. 1987. New York: Oxford University Press;102–117.
24. Menzel R, Kaisaki PJ, Rjasanowski I, Heinke P, Kerner W, Menzel S. A low renal threshold for glucose in diabetic patients with a mutation in the hepatocyte nuclear factor-1alpha (HNF-1alpha) gene. Diabet Med. 1998. 15:816–820.

25. Gallichan M. Self monitoring of glucose by people with diabetes: evidence based practice. BMJ. 1997. 314:964–967.

26. Urakami T, Morimoto S, Nitadori Y, Harada K, Owada M, Kitagawa T. Urine glucose screening program at schools in Japan to detect children with diabetes and its outcome-incidence and clinical characteristics of childhood type 2 diabetes in Japan. Pediatr Res. 2007. 61:141–145.

27. Urakami T, Kubota S, Nitadori Y, Harada K, Owada M, Kitagawa T. Annual incidence and clinical characteristics of type 2 diabetes in children as detected by urine glucose screening in the Tokyo metropolitan area. Diabetes Care. 2005. 28:1876–1881.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download