Abstract
Results between different clinical laboratory measurement procedures (CLMP) should be equivalent, within clinically meaningful limits, to enable optimal use of clinical guidelines for disease diagnosis and patient management. When laboratory test results are neither standardized nor harmonized, a different numeric result may be obtained for the same clinical sample. Unfortunately, some guidelines are based on test results from a specific laboratory measurement procedure without consideration of the possibility or likelihood of differences between various procedures. When this happens, aggregation of data from different clinical research investigations and development of appropriate clinical practice guidelines will be flawed. A lack of recognition that results are neither standardized nor harmonized may lead to erroneous clinical, financial, regulatory, or technical decisions.
Standardization of CLMPs has been accomplished for several measurands for which primary (pure substance) reference materials exist and/or reference measurement procedures (RMPs) have been developed. However, the harmonization of clinical laboratory procedures for measurands that do not have RMPs has been problematic owing to inadequate definition of the measurand, inadequate analytical specificity for the measurand, inadequate attention to the commutability of reference materials, and lack of a systematic approach for harmonization. To address these problems, an infrastructure must be developed to enable a systematic approach for identification and prioritization of measurands to be harmonized on the basis of clinical importance and technical feasibility, and for management of the technical implementation of a harmonization process for a specific measurand.
Figures and Tables
Fig. 1
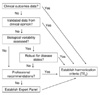
Concept of commutability of results for reference materials with results for a panel of individual clinical samples. (A) results for commutable reference materials that have the same numeric relationship between 2 measurement procedures as observed for a panel of patient samples. (B) results for noncommutable reference materials that have a different numeric relationship between 2 measurement procedures than observed for the patient samples.

Fig. 2
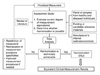
Proposed schema for developing method harmonization criteria based on Kenny et al. (31). TEa derived from allowable bias and imprecision, for the agreement of results among different measurement procedures.
References
1. Kohn LT, Corrigan JM, editors. To err is human: building a safer health system. 2000. Washington (DC): National Academy Press;287.
3. ISO. In vitro diagnostic medical devices-measurement of quantities in biological samples-metrological traceability of values assigned to calibrators and control materials. 2003. 1st ed. Geneva: ISO;ISO 17511:2003(E).
4. Armbruster D, Miller RR. The Joint Committee for Traceability in Laboratory Medicine (JCTLM): a global approach to promote the standardisation of clinical laboratory test results. Clin Biochem Rev. 2007. 28:105–113.
5. Panteghini M. Traceability as a unique tool to improve standardization in laboratory medicine. Clin Biochem. 2009. 42:236–240.

7. Thienpont LM. Accuracy in clinical chemistry: who will kiss Sleeping Beauty awake? Clin Chem Lab Med. 2008. 46:1220–1222.
8. Zegers I, Keller T, Schreiber W, Sheldon J, Albertini R, Blirup-Jensen S, et al. Characterization of the new serum protein reference material ERM-DA470k/IFCC: value assignment by immunoassay. Clin Chem. 2010. 56:1880–1888.

9. Little RR, Rohlfing CL, Tennill AL, Madsen RW, Polonsky KS, Myers GL, et al. Standardization of C-peptide measurements. Clin Chem. 2008. 54:1023–1026.

10. Stephan C, Klaas M, Müller C, Schnorr D, Loening SA, Jung K. Interchangeability of measurements of total and free prostate-specific antigen in serum with 5 frequently used assay combinations: an update. Clin Chem. 2006. 52:59–64.

11. Miller WG, Thienpont LM, Van Uytfanghe K, Clark PM, Lindstedt P, Nilsson G, et al. Toward standardization of insulin immunoassays. Clin Chem. 2009. 55:1011–1018.

12. Thienpont LM, Van Uytfanghe K, Beastall G, Faix JD, Ieiri T, Miller WG, et al. Report of the IFCC Working Group for Standardization of Thyroid Function Tests; part 1: thyroid-stimulating hormone. Clin Chem. 2010. 56:902–911.

13. Rose MP. Follicle stimulating hormone international standards and reference preparations for the calibration of immunoassays and bioassays. Clin Chim Acta. 1998. 273:103–117.
14. Tate JR, Bunk DM, Christenson RH, Katrukha A, Noble JE, Porter RA, et al. Standardisation of cardiac troponin I measurement: past and present. Pathology. 2010. 42:402–408.

15. Sturgeon CM, Berger P, Bidart JM, Birken S, Burns C, Norman RJ, et al. Differences in recognition of the 1st WHO international reference reagents for hCG-related isoforms by diagnostic immunoassays for human chorionic gonadotropin. Clin Chem. 2009. 55:1484–1491.

16. Thienpont LM, Van Houcke SK. Traceability to a common standard for protein measurements by immunoassay for in-vitro diagnostic purposes. Clin Chim Acta. 2010. 411:2058–2061.

17. Schimmel H, Zegers I, Emons H. Standardization of protein biomarker measurements: is it feasible? Scand J Clin Lab Invest Suppl. 2010. 242:27–33.

20. Vesper HW, Miller WG, Myers GL. Reference materials and commutability. Clin Biochem Rev. 2007. 28:139–147.
21. Caliendo AM, Shahbazian MD, Schaper C, Ingersoll J, Abdul-Ali D, Boonyaratanakornkit J, et al. A commutable cytomegalovirus calibrator is required to improve the agreement of viral load values between laboratories. Clin Chem. 2009. 55:1701–1710.

22. CLSI. CLSI document C53-A. Characterization and qualification of commutable reference materials for laboratory medicine: approved guideline. 2010. Wayne, (PA): CLSI.
23. Franzini C, Ceriotti F. Impact of reference materials on accuracy in clinical chemistry. Clin Biochem. 1998. 31:449–457.

24. Thienpont LM, Stockl D, Friedecky B, Kratochvila J, Budina M. Trueness verification in European external quality assessment schemes: time to care about the quality of the samples. Scand J Clin Lab Invest. 2003. 63:195–202.

25. Miller WG. Specimen materials, target values and commutability for external quality assessment (proficiency testing) schemes. Clin Chim Acta. 2003. 327:25–37.

26. Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig LM, et al. Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative. BMJ. 2003. 326:41–44.

27. Roddam AW, Price CP, Allen NE, Ward AM. Assessing the clinical impact of prostate-specific antigen assay variability and nonequimolarity: a simulation study based on the population of the United Kingdom. Clin Chem. 2004. 50:1012–1016.

28. NIH consensus statement on management of hepatitis C: 2002. NIH Consens State Sci Statements. 2002. 19:1–46.
29. Petersen PH, Sandberg S, Iglesias N, Sölétormos G, Aarsand AK, Brandslund I, et al. 'Likelihood-ratio' and 'odds' applied to monitoring of patients as a supplement to 'reference change value' (RCV). Clin Chem Lab Med. 2008. 46:157–164.

30. Karon BS, Boyd JC, Klee GG. Glucose meter performance criteria for tight glycemic control estimated by simulation modeling. Clin Chem. 2010. 56:1091–1097.

31. Kenny D, Fraser CG, Hyltoft Petersen P, Kallner A. Strategies to set global analytical quality specifications in laboratory medicine. Consensus agreement. Scand J Clin Lab Invest. 1999. 59:475–585.
32. Fraser CG. Biological variation: from principles to practice. 2001. Washington (DC): AACC Press.
33. Braga F, Dolci A, Mosca A, Panteghini M. Biological variability of glycated hemoglobin. Clin Chim Acta. 2010. 411:1606–1610.

34. Miller WG, Bruns DE, Hortin GL, Sandberg S, Aakre KM, McQueen MJ, et al. Current issues in measurement and reporting of urinary albumin excretion. Clin Chem. 2009. 55:24–38.

35. Miller WG, Myers GL, Ashwood ER, Killeen AK, Wang E, Ehlers GW, et al. State of the art in trueness and inter-laboratory harmonization for 10 analytes in general clinical chemistry. Arch Pathol Lab Med. 2008. 132:838–846.

36. Palmer-Toy DE. Comparison of pooled fresh frozen serum to proficiency testing material in College of American Pathologists surveys: cortisol and immunoglobulin E. Arch Pathol Lab Med. 2005. 129:305–309.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download