Abstract
Background
Hepcidin has recently been known as a negative regulatory hormone of iron. Hepcidin precursor, pro-hepcidin has been used as a surrogate and reported to be related to iron deficiency. We investigated serum pro-hepcidin levels in patients with iron deficiency anemia (IDA), anemia of chronic disorder (ACD) and ACD concomitant iron deficiency (ACD/ID) to assess its usefulness as a marker of iron deficiency and examined whether its level is associated with anemia, iron status or inflammation profiles involved in the synthesis of hepcidin.
Methods
We enrolled 50 patients with IDA, 46 with ACD, 12 with ACD/ID and 60 healthy controls. Complete blood cell count, iron parameters (iron, TIBC, trasferrin saturation, ferritin), C-reactive protein (CRP) and serum pro-hepcidin were measured.
Results
Patients with iron deficiency, the IDA group and ACD/ID group had lower serum pro-hepcidin levels than healthy controls and the ACD group. The cutoff value of pro-hepcidin for detecting iron deficiency was 230 ng/mL (sensitivity 88.1%, specificity 51.2%). Patients with increased CRP showed higher mean pro-hepcidin level than those with normal CRP and the difference was significant in the IDA group (P=0.02). And serum pro-hepcidin level was positively correlated with CRP level (r=0.30, P=0.04) in the IDA group but not with hemoglobin.
Figures and Tables
Fig. 1
Serum prohepcidin levels in healthy control, anemia of chronic disorder (ACD), iron deficiency anemia (IDA), and ACD with iron deficiency (ACD/ID) groups. Data are depicted as lower quartile, median, and upper quartile (boxes) and minimum/maximum ranges (whiskers).

Fig. 2
Relationship between serum prohepcidin and C-reactive protein levels in patients with iron deficiency anemia.

Table 1
Laboratory data in different groups of patients with anemia
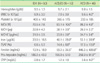
*P<0.05 when compared to IDA group; †P<0.05 when compared to ACD/ID group.
Abbreviations: IDA, iron deficiency anemia; ACD, anemia of chronic disorder; ACD/ID, anemia of chronic disorder with concomitant iron deficiency; WBC, white blood cell; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; TSAT, transferrin saturation; CRP, C-reactive protein.
Table 2
The correlation between laboratory parameters and serum pro-hepcidin levels in healthy control and anemia patients

Abbreviations: See Table 1.
References
1. Wish JB. Assessing iron status: beyond serum ferritin and transferrin saturation. Clin J Am Soc Nephrol. 2006. 1:S. S4–S8.

2. Fleming RE, Sly WS. Hepcidin: a putative iron-regulatory hormone relevant to hereditary hemochromatosis and the anemia of chronic disease. Proc Natl Acad Sci USA. 2001. 98:8160–8162.

3. Theurl I, Aigner E, Theurl M, Nairz M, Seifert M, Schroll A, et al. Regulation of iron homeostasis in anemia of chronic disorder and iron deficiency anemia: diagnostic and therapeutic implications. Blood. 2009. 113:5277–5285.
4. Nicolas G, Viatte L, Bennoun M, Beaumont C, Kahn A, Vaulont S. Hepcidin, a new iron regulatory peptide. Blood Cells Mol Dis. 2002. 29:327–335.

5. Nemeth E, Valore EV, Territo M, Schiller G, Lichtenstein A, Ganz T. Hepcidin, a putative mediator of anemia of inflammation, is a type II acute-phase protein. Blood. 2003. 101:2461–2463.

6. Nicolas G, Chauvet C, Viatte L, Danan JL, Bigard X, Devaux I, et al. The gene encoding the iron regulatory peptide hepcidin is regulated by anemia, hypoxia, and inflammation. J Clin Invest. 2002. 110:1037–1044.

7. Grebenchtchikov N, Geurts-Moespot AJ, Kroot JJ, den Heijer M, Tjalsma H, Swinkels DW, et al. High-sensitive radioimmunoassay for human serum hepcidin. Br J Haematol. 2009. 146:317–325.

8. Kulaksiz H, Gehrke SG, Janetzko A, Rost D, Bruckner T, Kallinowski B, et al. Pro-hepcidin: expression and cell specific localisation in the liver and its regulation in hereditary haemochromatosis, chronic renal insufficiency, and renal anaemia. Gut. 2004. 53:735–743.

9. Tsuchihashi D, Abe T, Komaba H, Fujii H, Hamada Y, Nii-Kono T, et al. Serum pro-hepcidin as an indicator of iron status in dialysis patients. Ther Apher Dial. 2008. 12:226–231.

10. Orhon FS, Ulukol B, Hanoluk A, Akar N. Serum pro-hepcidin levels in infant with iron deficiency anaemia. Int J Lab Hematol. 2008. 30:546–547.
11. Sasu BJ, Li H, Rose MJ, Arvedson TL, Doellgast G, Molineux G. Serum hepcidin but not prohepcidin may be an effective marker for anemia of inflammation (AI). Blood Cells Mol Dis. 2010. 45:238–245.

12. Kato A, Tsuji T, Luo J, Sakao Y, Yasuda H, Hishida A. Association of prohepcidin and hepcidin-25 with erythropoietin response and ferritin in hemodialysis patients. Am J Nephrol. 2008. 28:115–121.

13. Ryu EY, Jang SH, Yeom JS, Park ES, Seo JH, Lim JY, et al. Correlation between pro-hepcidin level and iron parameter in infant patients with iron deficiency anemia and anemia-free infants. Clin Pediatr Hematol-Oncol. 2007. 14:145–150.
14. Paesano R, Berlutti F, Pietropaoli M, Pantanella F, Pacifici E, Goolsbee W, et al. Lactoferrin efficacy versus ferrous sulfate in curing iron deficiency and iron deficiency anemia in pregnant women. Biometals. 2010. 23:411–417.

15. Theurl I, Mattle V, Seifert M, Mariani M, Marth C, Weiss G. Dysregulated monocyte iron homeostasis and erythropoietin formation in patients with anemia of chronic disease. Blood. 2006. 107:4142–4148.

17. Looker AC, Dallman PR, Carroll MD, Gunter EW, Johnson CL. Prevalence of iron deficiency in the United States. JAMA. 1997. 277:973–976.

18. Ganz T, Olibina G, Girelli D, Nemeth E, Westerman M. Immunoassay for human serum hepcidin. Blood. 2008. 112:4292–4297.

19. Kemna EH, Tjalsma H, Podust VN, Swinkels DW. Mass spectrometry-based hepcidin measurement in serum and urine: analytical aspects and clinical implication. Clin Chem. 2007. 53:620–628.

20. Lasocki S, Baron G, Driss F, Westerman M, Puy H, Boutron I, et al. Diagnostic accuracy of serum hepcidin for iron deficiency in critically ill patients with anemia. Intensive Care Med. 2010. 36:1044–1048.

21. Cheng PP, Jiao XY, Wang XH, Lin JH, Cai YM. Hepcidin expression in anemia of chronic disease and concomitant iron-deficiency anemia. Clin Exp Med. 2011. 11:33–42.

23. Punnonen K, Irjala K, Rajamaki A. Serum transferrin receptor and its ratio to serum ferritin in the diagnosis of iron deficiency. Blood. 1997. 89:1052–1057.

24. Dallalio G, Fleury T, Means RT. Serum hepcidin in clinical specimens. Br J Haematol. 2003. 122:996–1000.

25. Malyszko J, Malyszko JS, Hryszko T, Pawlak K, Mysliwiec M. Is hepcidin a link between anemia, inflammation and liver function in hemodialyzed patients? Am J Nephrol. 2005. 25:586–590.

26. Ulukol B, Orhon FS, Hanoluk A, Akar N. Serum pro-hepcidin levels and relationship with ferritin in healthy non-anaemic infants. Acta Haematol. 2007. 118:70–72.

27. Kim HR, Kim KW, Yoon SY, Kim SH, Lee SH. Serum pro-hepcidin could reflect disease activity in patients with rheumatoid arthritis. J Korean Med Sci. 2010. 25:348–352.

28. Chung J. Relationship between serum pro-hepcidin concentration and body iron status in female college students. Korean J Nutr. 2005. 38:750–755.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download