Abstract
Background
Accuracy of laboratory test results is an important issue. New guidelines for specimen delivery systems are needed for appropriate pretreatment of specimens and accuracy of results.
Methods
We evaluated various laboratory profiles, comparing the effects of specimen rack holders and coolants within transport containers. The hematology profiles (complete blood cell count [CBC], erythrocyte sedimentation rate [ESR]), chemistry profiles (aspartate aminotransaminase [AST], alanine aminotransaminase [ALT], gamma-glutamyl transferase [γ-GT], electrolytes [Na, K, Cl], glucose, lactate dehydrogenase [LD], creatinine kinase [CK]), and coagulation profiles (prothrombin time [PT], activated partial thromboplastin time [aPTT], fibrinogen level). We also investigated the effects of transportation time including the presence or absence of hemolyzation. We received from 9 different university hospital laboratories using conventional transporation methods.
Results
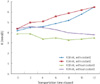
Hemolytic features were seen in short drawn specimens. Fewer result variations were observed in specimens transported with coolants. Average specimen transportation time was 11.3 hours, and average temperatures of container was 10.9℃ with coolant and 25.0℃ without coolants. Non-centrifuged specimens transported with coolants showed increased serum K levels than centrifuged specimens. Coagulation tests showed less than a 10% differences. Centrifuged specimen prior to transportation showed no hemolyzation and no differences in results.
Conclusions
Appropriate temperatures for each analyte should be defined to ensure the accuracy of results. To reduce hemolyzation, appropriate temperature and rack holder should be used. Temperature of the transport container should be monitored in objectively. Coagulation tests should be added as referral tests, if appropriate specimen transport monitoring system for time and temperature could be adopted.
Figures and Tables
Table 4
Temperature changes (℃) observed according to transportation time and external temperature of the transport vehicle
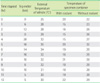
Table 5
Average transport time (hours) between participating laboratories and the central laboratory of reference laboratory
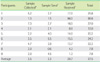
Table 7
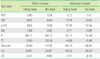
Average CBC & ESR result differences (%) of between participants' and reference laboratories

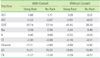
Table 8
Average blood chemistry (SST) test result differences (%) between participants' and reference laboratories
References
1. Berg B, Estborn B, Tryding N. Stability of serum and blood constituents during mail transport. Scand J Clin Lab Invest. 1981. 41:425–430.

2. Jensen EA, Stahl M, Brandslund I, Grinsted P. Stability of heparin blood samples during transport based on defined pre-analytical quality goals. Clin Chem Lab Med. 2008. 46:225–234.

3. Sinclair D, Briston P, Young R, Pepin N. Seasonal pseudohyperkalaemia. J Clin Pathol. 2003. 56:385–388.

4. Lewis SM, Bain BJ, editors. Practical haematology. 2001. 9th ed. Philadelphia: Churchill Livingstone.
5. Plebani M. Errors in clinical laboratories or errors in laboratory medicine? Clin Chem Lab Med. 2006. 44:750–759.

6. Stroobants AK, Goldschmidt HM, Plebani M. Error budget calculations in laboratory medicine: linking the concepts of biological variations and allowable medical errors. Clin Chim Acta. 2003. 333:169–176.

7. Lippi G, Guidi GC, Mattituzzi C, Plebani M. Preanalytical variability: the dark side of the moon in laboratory testing. Clin Chem Lab Med. 2006. 44:358–365.

8. Seamark D, Backhouse S, Barber P, Hichens J, Salzmann M, Powell R. Transport and temperature effects on measurement of serum and plasma potassium. J R Soc Med. 1999. 92:339–341.

9. Clinical and Laboratory Standards Institute. CLSI document H21-A4. Collection, transport, and processing of blood specimens for testing plasma-based coagulation assays; approved guideline. 2003. 4th ed. Wayne, PA: Clinical and Laboratory Standards Institute.




 PDF
PDF ePub
ePub Citation
Citation Print
Print







 XML Download
XML Download