Abstract
Background
For the diagnosis of tuberculosis (TB), a variety of tests based on the patients' immune response has been introduced. We evaluated the clinical usefulness of combined anti-tuberculosis antibody (anti-TB Ab) test and Interferon-γ release assay (IGRA), evaluating humoral and cellular immune response to Mycobacterium tuberculosis, respectively.
Methods
Among patients tested for IGRA, 78 patients diagnosed as TB and treated with anti-TB drug and 80 non-TB patients were included in this study. We used QuantiFERON-TB GOLD (QFT, Cellestis limited, Australia) for IGRA and an immunochromatographic assay, Easy Test TB (ASAN PHARM, Korea), for anti-TB Ab test.
Results
The sensitivity, specificity, and positive and negative predictive values of Easy Test TB were 23.1%, 98.8%, 94.7% and 56.8%, respectively. QFT had a significantly higher sensitivity than Easy Test TB (67.9% vs. 23.1%; P<0.05). The agreement between the two assays was poor (69.6%, k=0.190). Of the 18 cases with positive Easy Test TB, six (33%) showed negative QFT results. The combination of Easy Test TB and QFT had a significantly higher sensitivity than single QFT (75.6%, vs. 67.9%; P=0.031).
Figures and Tables
 | Fig. 1Pictorial and diagrammatic representation of Easy Test TB device. (A) Photograph of Easy Test TB kit. Serum pad (S), control line (C) and test line (T) is marked on the device. (B) Diagram showing the inner strip details. Serum pad, conjugate pad, nitrocellulose membrane and absorbent pad are piled up one by one within the inner strip. |
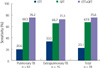 | Fig. 2The sensitivities of Easy Test TB, QFT and a combination of the two assays in patients with pulmonary and extrapulmonary TB.
Abbreviations: TB, tuberculosis; ETT, Easy Test TB; QFT, QuantiFERON-TB GOLD.
|
References
1. Dye C, Scheele S, Dolin P, Pathania V, Raviglione MC. Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. WHO Global Surveillance and Monitoring Project. JAMA. 1999. 282:677–686.
2. World Health Organization (WHO). WHO/HTM/TB/2009.426. Global tuberculosis control: a short update to the 2009 report. 2009. Geneva, Switzerland: World Health Organization.
3. Bartoloni A, Strohmeyer M, Bartalesi F, Messeri D, Tortoli E, Farese A, et al. Evaluation of a rapid immunochromatographic test for the serologic diagnosis of tuberculosis in Italy. Clin Microbiol Infect. 2003. 9:632–639.

4. Pfaller MA. Application of new technology to the detection, identification, and antimicrobial susceptibility testing of mycobacteria. Am J Clin Pathol. 1994. 101:329–337.

5. Chang HE, Heo SR, Yoo KC, Song SH, Kim SH, Kim HB, et al. Detection of Mycobacterium tuberculosis complex Using Real-time Polymerase Chain Reaction. Korean J Lab Med. 2008. 28:103–108.

6. Bothamley GH. Serological diagnosis of tuberculosis. Eur Respir J Suppl. 1995. 20:676s–688s.
7. Chan ED, Heifets L, Iseman MD. Immunologic diagnosis of tuberculosis: a review. Tuber Lung Dis. 2000. 80:131–140.

8. Steingart KR, Henry M, Laal S, Hopewell PC, Ramsay A, Menzies D, et al. A systematic review of commercial serological antibody detection tests for the diagnosis of extrapulmonary tuberculosis. Postgrad Med J. 2007. 83:705–712.

9. Steingart KR, Henry M, Laal S, Hopewell PC, Ramsay A, Menzies D, et al. Commercial serological antibody detection tests for the diagnosis of pulmonary tuberculosis: a systematic review. PLoS Med. 2007. 4:e202.

10. Pai M, Kalantri S, Dheda K. New tools and emerging technologies for the diagnosis of tuberculosis: part I. Latent tuberculosis. Expert Rev Mol Diagn. 2006. 6:413–422.

11. Zellweger JP, Zellweger A, Ansermet S, de Senarclens B, Wrighton-Smith P. Contact tracing using a new T-cell based test: better correlation with tuberculosis exposure than the tuberculin skin test. Int J Tuberc Lung Dis. 2005. 9:1242–1247.
12. Raja A. Immunology of tuberculosis. Indian J Med Res. 2004. 120:213–232.
13. Glatman-Freedman A, Casadevall A. Serum therapy for tuberculosis revisited: reappraisal of the role of antibody-mediated immunity against Mycobacterium tuberculosis. Clin Microbiol Rev. 1998. 11:514.

14. Doherty TM, Andersen P. Vaccines for tuberculosis: novel concepts and recent progress. Clin Microbiol Rev. 2005. 18:687.

15. Torres M, Mendez-Sampeiro P, Jimenez-Zamudio L, Teran L, Camarena A, Quezada R, et al. Comparison of the immune response against Mycobacterium tuberculosis antigens between a group of patients with active pulmonary tuberculosis and healthy household contacts. Clin Exp Immunol. 1994. 96:75–78.

16. Del Prete R, Picca V, Mosca A, D'Alagni M, Miragliotta G. Detection of anti-lipoarabinomannan antibodies for the diagnosis of active tuberculosis. Int J Tuberc Lung Dis. 1998. 2:160–163.
17. Harboe M, Wiker HG. The 38-kDa protein of Mycobacterium tuberculosis: a review. J Infect Dis. 1992. 166:874–884.
18. Nagai S, Wiker HG, Harboe M, Kinomoto M. Isolation and partial characterization of major protein antigens in the culture fluid of Mycobacterium tuberculosis. Infect Immun. 1991. 59:372–382.

19. Kox LF. Tests for detection and identification of mycobacteria. How should they be used? Respir Med. 1995. 89:399–408.

20. Yoshikawa M, Yoneda T, Tsukaguchi K, Narita N. Diagnosis of mycobacterial disease by biochemical and immunological parameters. Nippon Rinsho. 1998. 56:3057–3061.
21. Steingart KR, Ramsay A, Pai M. Commercial serological tests for the diagnosis of tuberculosis: do they work? Future Microbiol. 2007. 2:355–359.

22. Ongut G, Ogunc D, Gunseren F, Ogus C, Donmez L, Colak D, et al. Evaluation of the ICT Tuberculosis test for the routine diagnosis of tuberculosis. BMC Infect Dis. 2006. 6:37.

23. Cole RA, Lu HM, Shi YZ, Wang J, De-Hua T, Zhou AT. Clinical evaluation of a rapid immunochromatographic assay based on the 38 kDa antigen of Mycobacterium on patients with pulmonary tuberculosis in China. Tuber Lung Dis. 1996. 77:363–368.

24. Kim MY, Ha GY, Jung DG, Song KE, Suh JS, Lee WK, et al. Clinical Usefulness of An Immunochromatographic Assay Based on 38 kDa Antigen for The Diagnosis of Active Tuberculosis. Korean J Clin Pathol. 1999. 19:647–656.
25. Senol G, Erer OF, Yalcin YA, Coskun M, Gündüz AT, Biçmen C, et al. Humoral immune response against 38-kDa and 16-kDa mycobacterial antigens in tuberculosis. Eur Respir J. 2007. 29:143–148.

26. Okuda Y, Maekura R, Hirotani A, Kitada S, Yoshimura K, Hiraga T, et al. Rapid serodiagnosis of active pulmonary Mycobacterium tuberculosis by analysis of results from multiple antigen-specific tests. J Clin Microbiol. 2004. 42:1136–1141.

28. Thybo S, Richter C, Wachmann H, Maselle SY, Mwakyusa DH, Mtoni I, et al. Humoral response to Mycobacterium tuberculosis-specific antigens in African tuberculosis patients with high prevalence of human immunodeficiency virus infection. Tuber Lung Dis. 1995. 76:149–155.

29. World Health Organization (WHO). WHO tuberculosis diagnositcs workshop: product development guidelines, Workshop report. 1997. Geneva, Switzerland: World Health Organization;1–27.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download