Abstract
Minimal change disease (MCD) is a common cause of nephrotic syndrome and relatively well responds with steroid treatment. However, nearly half of patients with MCD experience recurrence of nephrotic syndrome. Thromboembolic events including renal vein thrombosis may occur in patients with MCD, but portal vein thrombosis rarely occurs. We experienced a case of frequent relapse/steroid dependent MCD with nephrotic syndrome progressed to steroid resistance associated with portal vein thrombosis. This patient showed complete remission of MCD and resolution of portal vein thrombosis after treatment with corticosteroid, cyclosporine, mycophenolate mofetil, and anticoagulant.
Nephrotic syndrome is a disease characterized by albuminuria, hypoalbuminemia, edema, and hyperlipidemia, and thrombus and embolism are major complications of nephrotic syndrome. The location of the thrombus varies among reports, but it mostly occurs in the vein, with renal vein thrombosis being most common; it also occurs in the deep vein or artery of the pelvic limbs.12 Thrombosis is a complication of adult nephrotic syndrome, which mainly occurs in membranous glomerulonephritis, and its occurrence is relatively lower in minimal change disease.1
Minimal change disease shows good response to steroid treatment, and 85% – 90% of patients show complete remission of albuminuria.3 However, there is relapse in 56% – 76% of adult patients that show a response to steroids, while 10% – 25% show frequent relapse.4 Treatment of recurrent minimal change disease is achieved by first using an alkylating agent (cyclophosphamide), as well as other drugs such as calcineurin inhibitors (cyclosporine or tacrolimus), mycophenolate mofetil (MMF), and rituximab, but there is still no clearly established treatment protocol.3
The author experienced a case in which a patient with frequently relapsing minimal change disease, who also showed steroid resistance and the clinical features of hepatic portal vein thrombosis, went into complete remission with the three treatments of steroids, cyclosporine, and MMF. Following this course, the hepatic portal vein thrombosis also disappeared. Thus, the case will be reported along with a literature review.
Chief Complaint: Edema and Hydrops Abdominis.
History of presenting illness: The patient was hospitalized in October 2003 with chief complaints of albuminuria, hypoalbuminemia, edema, and hyperlipidemia, and he was diagnosed with minimal change disease following renal biopsy (Fig. 1A). He showed complete remission at 4 weeks after using 60 mg (1 mg/kg/day) of prednisolone (Solondo®; Yuhan Corporation, Seoul, Korea); he showed side effects during dose reduction such as blushing. Thus, the drug was switched to deflazacort (Calcort®; Handok Inc., Seoul, Korea), which was continuously taken. After 6 months of treatment, the patient showed complete remission of albuminuria, and thus he discontinued his intake of deflazacort. Two months later in June 2004, the first relapse occurred with at least 2 g/g for the spot urine protein/creatinine ratio, and thus the patient took 60 mg of deflazacort and then reached complete remission. Later, the patient's steroid intake was randomly discontinued and the patient showed a second relapse with a spot urine protein/creatinine ratio of 5.97 g/g in April 2005. The patient showed remission using 72 mg of deflazacort, after which 100 mg (50 mg, twice daily) of low-dose steroid and cyclosporine (Cipol-N®; Chong Kun Dang Pharmaceutical Corp., Seoul, Korea) were added for maintenance therapy. However, the patient did not take the drugs very well, so the cyclosporine was discontinued while he was maintained on a low-dose steroid and deflazacort at 12 mg/every other day. The spot urine protein/creatinine ratio in December 2006 and October 2007 was 12.42 g/g and 5.24 g/g, respectively, which were thus indicative of the third and fourth relapse episodes. Therefore, the combination therapy of deflazacort and cyclosporine was maintained. Most of the relapses were caused by the patient's arbitrary discontinuity of medication. After the fifth relapse in April 2010, with a spot urine protein/creatinine ratio of 11.51 g/g, another relapse occurred multiple times at 3–6-month intervals; thus, a renal biopsy was performed again in December 2012. The results showed no focal segmental glomerulosclerosis aside from minimal change disease (Fig. 1B, 1C, and 1D). Later, while observing the patient's progress on an outpatient basis, he was hospitalized due to hydrops abdominis and severe edema in November 2013.
Family History: No Significant Findings.
Findings and laboratory opinions: At the time the patient was hospitalized, his height was 159 cm and his weight was 68 kg, which was 8 kg more than at the first medical examination. The patient presented with hydrops abdominis and edema, and his face was round, with a moon-like shape. The patient's blood pressure was 105/60 mmHg, and he had a pulse of 80 beats/minute, a body temperature of 36.5℃, and a respiration rate of 20 breaths/minute. The heart sound was clear and regular, and the chest findings were normal. He had no oppressive pain in either of his kidneys, and he exhibited pitting edema that was more severe than moderate in both pelvic limbs.
On peripheral blood examination, the patient's hemoglobin was 15.9 g/dL, hematocrit 45.2%, and leucocytes 8,780/mm3. During the serum electrolyte test, the patient's sodium was 136 mmol/L, potassium 4.6 mmol/L, chlorine 107 mmol/L, calcium 7.0 mg/dL, and phosphorous 5.2 mg/dL. During the serum biochemistry test, the patient's blood urea nitrogen was 17 mg/dL, creatinine 0.8 mg/dL, glomerular filtration rate 124.2 mL/min/1.73m2, aspartate aminotransferase 30 U/L, alanine aminotransferase 22 U/L, alkaline phosphatase 250 U/L, and total bilirubin 0.5 mg/dL. The total cholesterol increased to 427 mg/dL and the albumin decreased to 1.6 g/dL. Antithrombin Ⅲ was 49.3% (normal range: 70%–130%), protein C was 158% (normal range: 70%–140%), and protein S was 102% (normal range: 60%–130%). With respect to the urinalysis, protein 3+, the erythrocyte count was 10–14 high-power fields (HPF), and the spot urine protein/creatinine ratio was 8.05 g/g. Chest radiograph showed normal results.
Treatment and clinical course: A diuretic was used to control the edema and hydrops abdominis, and 60 mg of prednisolone was used for at least 4 weeks to treat the relapse of minimal change disease; however, there was no change in the amount of albuminuria. Cyclophosphamide was about to be used, but the patient refused due to concerns of its side effects. On the abdominal ultrasound that was performed to check for hydrops abdominis, hepatic portal vein thrombosis was discovered, and thus the patient began to receive treatment using heparin and warfarin, which was adjusted within the prothrombin time range of 2–3 INR (International Normalized Range) (Fig. 2A). Due to thrombosis, prednisolone was reduced to 0.5 mg/kg/day, and the patient began to take 100 mg of cyclosporine twice a day. When using the steroid exclusively, the edema continued even after taking a high-dose furosemide intravenous injection, but it was somewhat controlled after adding cyclosporine; thus, the patient was discharged. Severe albuminuria continued and the albumin index fell to below 2.0 g/dL, even after using cyclosporine for 4 weeks; thus, 500 mg of MMF (Cellcept®; Roche Korea, Seoul, Korea) was added twice a day while gradually reducing the steroid. After using MMF for 4 weeks, the spot urine protein/creatinine ratio decreased to 2.25 g/g, so the steroid use was further reduced; 2 weeks later, the patient reached complete remission. Since then, he has been receiving combination therapy consisting of three drugs; he started taking 4.5 mg of deflazacort 1 time/day, 100 mg of cyclosporine 2 times/day, and 250 mg of MMF 2 times/day. He has been showing stable progress without relapse for at least a year thus far (Fig. 3A and 3B). The hepatic portal vein thrombus was completely gone as per the abdominal ultrasound taken in March 2015, and warfarin was discontinued a year and a half after beginning the thrombosis treatment (Fig. 2B).
Minimal change disease responds excellently to steroids as a key factor of nephrotic syndrome in both adults and children. Adults show slower responses to steroids than do children, and thus 85% of children show remission 8 weeks after beginning treatment, while only approximately 50% of adults show remission.5 Approximately 50%–60% of patients with minimal change disease that reach complete remission end up experiencing relapse. Relapse is related to the patient's age and the period of steroid intake, and recurrence rates are comparably lower in adults than in children.6 In this case, the patient had low compliance with his medication and he discontinued treatment by himself, thereby showing frequent relapse. He also showed resistance to the drugs in 2013 when he was hospitalized, showing no response even after using a steroid and cyclosporine for more than 8 weeks. Therefore, it is important to warn those patients with minimal change disease to always comply with their prescribed medication.
Steroid is increased, or cyclophosphamide, cyclosporine, MMF, and rituximab are administered, when the first relapse occurs, but there are still not enough clinical studies to support this. Cyclophosphamide has a longer maintenance period of remission than cyclosporine, and it was shown to be effective for the patient who had trouble enduring the steroid; however, it may cause severe cytotoxicity such as sterility, myelosuppression, infection, and malignant tumors.7 When treating steroid-dependent minimal change disease, cyclosporine shows a similar remission rate as cyclophosphamide, but it has fewer side effects.8 Eguchi et al. reported that patients with minimal change disease, and those who showed relapse, can reach remission more quickly if given a combination of cyclosporine and steroid rather than being given steroid alone.9 This study shows that the combination therapy of steroid and cyclosporine can be presented as an alternative that reduces the side effects associated with long-term steroid use. This case also considered side effects such as sterility, as the patient is young, and thus a combination therapy featuring a steroid and cyclosporine was used instead of cyclophosphamide. However, following the combination therapy of cyclosporine, the patient showed repeated relapse; thus, a renal biopsy was performed again to verify whether a new occurrence of focal segmental glomerulosclerosis was present rather than minimal change disease. However, the biopsy findings showed that the patient presented with minimal change disease. As such, it is necessary to assess whether focal segmental glomerulosclerosis has newly occurred in cases where patients show frequent relapse of minimal change disease, or if they are showing secondary resistance to steroid treatment after repeated relapses.10
Even though this case showed no other findings aside from minimal change disease during renal biopsy, severe hypoalbuminosis and albuminuria continued during the combination therapy that included cyclosporine; thus, MMF was added. MMF is a drug that has been used since the 1990s to prevent rejection following kidney transplantation, and many recent studies have shown the effects of MMF on glomerulonephritis. However, most of these investigations are small-scale retrospective studies that do not clearly detail the role of MMF in treating glomerulus diseases.11 Clara et al. revealed that MMF is useful for patients with constantly relapsing minimal change disease despite the use of steroids, cyclophosphamide, and cyclosporine, but the researchers reported that it is unclear whether this combination reduces relapse after treatment termination.12 Zhao et al. further discovered that MMF has fewer side effects than cyclophosphamide and cyclosporine; it also enables the successful reduction of steroids in steroid-dependent glomerulus diseases, reduces this dependency, and also plays a significant role in reducing the side effects associated with the long-term use of steroids.7 Moreover, if MMF is administered to those pediatric patients with steroid-dependent nephrotic syndrome who are under treatment with a steroid and cyclosporine, then the amount of steroid and cyclosporine can be reduced, and the recurrence rate also decreases.13 This case also used cyclosporine and steroids along with MMF, thereby inducing quick remission and reducing the use of steroids at an early stage.
The mechanism that causes thrombosis, which is a major complication of nephrotic syndrome, is not clear, but various factors seem to be associated with its development. The genetic factors that cause thrombus embolism are accompanied by risk factors such as inflammation, drugs, and central vein catheterization, thereby causing thrombus. Moreover, antithrombin and protein S, which are important proteins involved in coagulation control due to a deficiency of glomerulus, are lost, and the hemostasis protein can be generated in the liver to maintain a balance between this protein loss and synthesis, ultimately affecting thrombus development.14 The platelet count and activity increased in pediatric patients with nephrotic syndrome, and there is also the excessive coagulation of blood platelets, the secretion of active materials, and an increased expression of activity signs on the surface of the blood platelets. Accordingly, it is assumed that the blood platelets may be involved in the occurrence of a thrombus in the vein and artery that occurs in nephrotic syndrome. However, the correlation between blood platelet variation and thrombosis occurrence in nephrotic syndrome is unclear, and preventive antithrombotic treatment is not yet recommended.15 The treatment of thrombosis that occurred in nephrotic syndrome is equivalent to the treatment of other types of thrombosis, such as using heparin first and then switching to warfarin. There are no principles for the required treatment period, but warfarin is maintained in most cases, as long as there are clinical findings of nephrotic syndrome. The patient in this case took warfarin for a year and a half after the occurrence of hepatic portal vein thrombosis in November 2013, and he was only able to stop taking warfarin after verifying that there were no additional clinical symptoms and that the hepatic portal vein thrombus improved on ultrasound. It is important to suspect that those high-risk patients that show severe nephrotic syndrome, and those that are resistant to treatment, may have phlebothrombosis.16
The patient showed resistance to the steroid, and thus a combination therapy of steroid and cyclosporine was used as a secondary drug, but the patient showed no response. Therefore, MMF was added as an alternative option, and the phlebothrombosis was completely gone following complete remission. The results of this study were reported in association with a literature review.
Figures and Tables
Fig. 1
Renal histologic finding.
A. Light microscopy. ×400, PAS (October, 2003). A glomerulus is normal in size and shows normal cellularity.
B. Light microscopy. ×40, PAS (December, 2012). There is no focal and segmental glomerulosclerosis on lots of glomerulus.
C. Light microscopy. ×400, PAS (December, 2012). Compared to the previous biopsy (October, 2003), there is no interval change.
D. Electron microscopy. (December, 2012). The electron microscopic examination shows effaced foot process of epithelial cell (arrow).
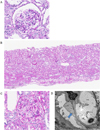
Fig. 2
Abdominal ultrasonography image.
A. Internal hyperechoic material suggesting a portal vein thrombosis was shown on main portal vein (December, 2013).
B. Regression of portal vein thrombosis with normal portal venous flow was shown on doppler ultrasonography (March, 2015).
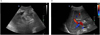
Fig. 3
Long-term treatment course of steroid resistant minimal change disease.
A. Treatment with steroid, cyclosporine, and mycophenolate mofetil (MMF) resulted in complete remission.
B. Changes of urine protein to creatinine ratio and serum albumin levels. The left side Y-axis shows urine protein to creatinine ratio (g/g), while the right side Y-axis shows albumin levels (g/dL).

References
1. Singhal R, Brimble KS. Thromboembolic complications in the nephrotic syndrome: pathophysiology and clinical management. Thromb Res. 2006; 118:397–407.

2. Mahmoodi BK, ten Kate MK, Waanders F, Veeger NJ, Brouwer JL, Vogt L, et al. High absolute risks and predictors of venous and arterial thromboembolic events in patients with nephrotic syndrome: results from a large retrospective cohort study. Circulation. 2008; 117:224–230.

3. Hogan J, Radhakrishnan J. The treatment of minimal change disease in adults. J Am Soc Nephrol. 2013; 24:702–711.

4. Nakayama M, Katafuchi R, Yanase T, Ikeda K, Tanaka H, Fujimi S. Steroid responsiveness and frequency of relapse in adult-onset minimal change nephrotic syndrome. Am J Kidney Dis. 2002; 39:503–512.

5. Waldman M, Crew RJ, Valeri A, Busch J, Stokes B, Markowitz G, et al. Adult minimal-change disease: clinical characteristics, treatment, and outcomes. Clin J Am Soc Nephrol. 2007; 2:445–453.

6. Korbet SM, Schwartz MM, Lewis EJ. Minimal-change glomerulopathy of adulthood. Am J Nephrol. 1988; 8:291–297.

7. Zhao M, Chen X, Chen Y, Liu Z, Liu Y, Lu F, et al. Clinical observations of mycophenolate mofetil therapy in refractory primary nephrotic syndrome. Nephrology (Carlton). 2003; 8:105–109.

8. Ponticelli C, Edefonti A, Ghio L, Rizzoni G, Rinaldi S, Gusmano R, et al. Cyclosporine versus cyclophosphamide for patients with steroid-dependent and frequently relapsing idiopathic nephrotic syndrome: a multicenter randomized controlled trial. Nephrol Dial Transplant. 1993; 8:1326–1332.
9. Eguchi A, Takei T, Yoshida T, Tsuchiya K, Nitta K. Combined cyclosporine and prednisolone therapy in adult patients with the first relapse of minimal-change nephrotic syndrome. Nephrol Dial Transplant. 2010; 25:124–129.

10. Mak SK, Short CD, Mallick NP. Long-term outcome of adult-onset minimal-change nephropathy. Nephrol Dial Transplant. 1996; 11:2192–2202.

11. Shigidi MM. The treatment of relapse in adults with minimal change nephrotic syndrome: myths and facts. Saudi J Kidney Dis Transpl. 2011; 22:10–17.
12. Day CJ, Cockwell P, Lipkin GW, Savage CO, Howie AJ, Adu D. Mycophenolate mofetil in the treatment of resistant idiopathic nephrotic syndrome. Nephrol Dial Transplant. 2002; 17:2011–2013.

13. Fujinaga S, Ohtomo Y, Umino D, Takemoto M, Shimizu T, Yamashiro Y, et al. A prospective study on the use of mycophenolate mofetil in children with cyclosporine-dependent nephrotic syndrome. Pediatr Nephrol. 2007; 22:71–76.

14. Kerlin BA, Ayoob R, Smoyer WE. Epidemiology and pathophysiology of nephrotic syndrome-associated thromboembolic disease. Clin J Am Soc Nephrol. 2012; 7:513–520.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download