Abstract
Protein losing enteropathy (PLE) due to systemic lupus erythematosus (SLE) is relatively uncommon. PLE may be appeared sequentially after the diagnosis of SLE or concurrently with SLE. In most of concurrent cases, PLE was diagnosed one of various symptoms of SLE. Cases of PLE as the initial and only clinical presentation of SLE have been rarely reported. We described a 30-year old woman with general edema and abdominal distension was diagnosed PLE after stool alpha 1 antitrypsin clearance test. Her symptoms were getting worse even though the treatment with intravenous albumin. She was finally diagnosed PLE associated with SLE by additional laboratory findings (positive antinuclear antibody and anti-dsDNA IgG and low C3, C4 and CH50). She was treated with high dose of steroids and her symptoms were improved.
Protein losing enteropathy (PLE) is a clinical entity characterized by hypoalbuminemia secondary to excessive loss of serum proteins from gastrointestinal (GI) tract. It can be caused by many GI disorders like ulcerative colitis, celiac sprue, intestinal lymphangiectasia, congestive cardiac failure and GI malignancy.1 The mechanisms of protein loss through GI tract include mucosal ulcerations, increased mucosal permeability, and lymphatic obstruction.2 Histopathological changes in duodenum and colon are non specific.3 Protein loss through GI tract can be confirmed by 99mTc-labeled human serum albumin scintigraphy (HAS) and stool alpha 1 antitrypsin (A1AT) clearance.4 PLE due to systemic lupus erythematosus (SLE) is relatively uncommon and less than 70 cases in the world and only 6 cases in Korea have been reported in the literature.5678910 PLE may be appeared sequentially after the diagnosis of SLE or concurrently with SLE. In most of concurrent cases, PLE was diagnosed one of various symptoms of SLE. Cases of PLE as the initial and only clinical presentation of SLE have been rarely reported. In Korea, PLE as the solitary symptom associated with SLE was reported only in a case.7 Therefore, we report that a case of PLE as the only clinical manifestation of SLE.
A 30-year-old female was admitted to our hospital in August 2014 with a history of general edema and abdominal distension that started one month ago. On the initial examination, there were eyelid edema, both leg pitting edema and abdominal distension without tenderness or pain. There was no musculoskeletal symptom and mucocutaneous lesion. She had a chronically ill-looking appearance. Her blood pressure was 120/80 mmHg, heart rate was 80 per minute, and respiratory rate was 20 per minute. Initial laboratory studies were as follows: white blood cell count 4610/㎣, hemoglobin 12.4 g/dL, and platelet count 241,000/㎣, aspartate aminotransferase 29 IU/L, alanine transaminase 17 IU/L, alkaline phosphatase 128 IU/L, lactate dehydrogenase 325 IU/L, total protein 4.6 ㎎/dL, albumin 2.0 g/dL, total bilirubin 0.1 ㎎/dL, creatinine 0.44 ㎎/dL. There was no hematuria and proteinuria in urinary analysis. Her chest radiography was normal. Abdominal computed tomography scan revealed diffuse bowel wall thickening with large amount of ascites (Fig. 1). In ascites analysis, there was no malignant cell and serum-ascites albumin gradient was 1.4. Gastrofibroscopy (GFS) and colon fibroscopy (CFS) was done to evaluate bowel wall edema and hypoalbuminemia. There were no specific lesions except general bowel edema in GFS and CFS (Fig. 2). For the diagnosis of PLE, stool A1AT clearance was tested and the result was 106 mL/24 hours (normal ≤ 27 mL/24 hours). 99mTc-labeled HAS was not available in our hospital. She was diagnosed with PLE and was treated with intravenous 20% albumin and low-fat, high-protein diet. However, albumin level was not increased and clinical symptoms were getting worse. To evaluate combined autoimmune disease, we checked autoimmune marker. Antinuclear antibody was positive (titer, 1:2560). C3, C4 and CH50 were 24 ㎎/dL (normal 90~180 ㎎/dL), 2 ㎎/dL (normal 10~40 ㎎/dL), and 6.6 U/mL (normal 23~46 U/mL) respectively. Anti-dsDNA IgG was 85 IU/mL (normal < 25 IU/mL). Anti-SSA/Ro antibody was positive. Anti-Smith antibody and Anti-SSB/La antibody were negative. Based on these laboratory findings and unknown origin PLE, we finally diagnosed PLE associated with SLE. She was treated with high dose steroid (methylprednisolone 50 ㎎ per day). During follow up, Malar rash was detected. Therefore she was diagnosed SLE definitely according to 2012 SLICC classification criteria for SLE.11 After 2 weeks, serum albumin level was increased to 3.6 ㎎/dL and symptoms were getting better. Now, one year after treatment, she is being treated with methylprednisolone (2 ㎎ per day), azathioprine (100 ㎎ per day), and hydroxychloroquine (200 ㎎ twice a day). Her albumin level was 4.1 g/dL at the latest visit (Fig. 3) and there were no any symptoms related SLE.
We reported a case of PLE that presented as the only clinical manifestation of SLE. In Korea, 6 cases of PLE associated with SLE have been reported. Only one of these cases is similar to our case in which PLE is presented as the solitary clinical manifestation of SLE. The 6 cases are presented in (Table 1).
There have been several reports of PLE associated with SLE since it was first described in 1969 by Waldman et al.12 Almost 70 cases have been reported up to now, however as our best of knowledge, only 6 cases were shown in the Korean literatures.5678910 PLE associated with SLE is occurred in any age and skewed female preponderance. Most patients with PLE with SLE show edema, hypoalbuminemia, and hypercholesterolemia. The diarrhea is also showed in 50% of the patients with SLE-related PLE.613
The exact pathogenesis of PLE associated with SLE is still unclear. Mucosal ulceration, non-necrotizing mesenteric or intestinal blood vessel vasculitis, increase in capillary permeability caused by intravascular activation and conversion of complement, cytokine or complement-mediated vascular or mucosal damage, and intestinal lymphangiectasia have been postulated as the pathogenic mechanisms.31314 In our case, biopsies at GFS and CFS were confirmed as chronic inflammations, and these were non-specific findings for the diagnosis.
The diagnosis of PLE associated with SLE depends on the exclusion of other causes of hypoalbuminemia and PLE. Stool A1AT can be used as a diagnostic test.4 A1AT is a protein which has a molecular weight similar to albumin. After being synthesized in the liver, it is neither actively secreted nor absorbed and is resistant to proteolysis or degradation in the gut. Therefore, estimation of 24 hours stool clearance indicates the loss through GI tract. Normal stool A1AT clearance is < 27 mL/24 hours. Moreover, 99mTc-labeled HAS can determine the location of the leakage with a high sensitivity.15 But it was not available in our hospital.
Corticosteroid and immunosuppressants (cyclophosphamide or azathioprine) were the mainstay treatment for PLE associated with SLE. Mok CC et al reported that 88% of patients had a good clinical recovery with high dose prednisolone for six months and then tapered with azathioprine.13 Chen Z et al reported that most of patients with PLE associated with SLE responded well to the combined therapy of prednisolone and cyclophosphamide.16 However, it remains unclear whether steroid monotherapy or combination therapy with immunosuppressants is more appropriate for PLE associated with SLE patients. In this case, patient was initially treated with high dose steroid and then tapered with azathioprine and achieved a good clinical response.
PLE is rarely associated with SLE, but as seen in our case, it can be presented as the solitary clinical manifestation of SLE. Therefore, physicians should be always kept this possibility in mind.
Figures and Tables
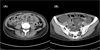 | Fig. 1Computed tomography scan of abdomen showed (A) diffuse bowel thickening (B) large amount of ascites. |
References
1. Henry J Binder. Disorders of absorption. In : Kasper , Fauci , Hauser , editors. Harrison's Principles of Internal Medicine. 19th edn. NewYork: McGrawHill;2015. p. 1945–1946.
2. Milovic V, Caspary WF, Stein J, et al. Protein-losing gastroenteropathy. Up To Date Gastroenterology [online]. Accessed 1 March 2005. Available at: http://www.uptodate.com/.
3. Kim YG, Lee Ck, Byeon JS, Myung SJ, Oh JS, Nah SS, et al. Serum cholesterol in idiopathic and lupus-related protein-losing enteropathy. Lupus. 2008; 17:575–579.

4. Tian XP, Zhang X. Gastrointestinal involvement in systemic lupus erythematosus: Insight into pathogenesis, diagnosis and treatment. World J Gastroenterol. 2010; 16:2971–2977.

5. Lee KN, Park YH, Lee SH, Lee JC, Lim MK, Cho YS, et al. A case of systemic lupus erythematosus with chylothorax, chronic interstitial cystitis and protein-losing enteropathy. Korean J Med. 2000; 59:555–560.
6. Yoon CO, Kim TH, Kang MS, Lee JI, Kang TY, Kim KC, et al. A Case of Protein-Losing Enteropathy Associated with Systemic Lupus Erythematosus. J Korean Rheum Assoc. 2001; 8:48–52.
7. Kim GW, Seo JS, Han SW, Kang YM. A Case of Systemic Lupus Erythematosus Presenting with Protein Losing Enteropathy. J Korean Rheum Assoc. 2003; 10:426–432.
8. Park JM, Ahn SY, Shin JI, Yun MJ, Lee JS. A systemic lupus erythematosus patient with protein losing Enteropathy. Yonsei Med J. 2004; 45:923–926.

9. Lee KH, Kwon CM, Kim HD, Yun DY, Lee JY, Hong YH, et al. A Case of Protein-losing Enteropathy Treated with High Dose Intravenous Glucocorticoid Therapy in Systemic Lupus Erythematosus. Yeungnam Univ J Med. 2005; 22:253–258.

10. Cho TH, Cho HR, Jeong KH, Haw CR. A Case of Annular Polycyclic Type of Subacute Cutaneous Lupus Erythematosus with Protein Losing Enteropathy. Korean J Dermatol. 2007; 45:735–738.
11. Petri M, Orbai AM, Alarcón GS, Gordon C, Merrill JT, Fortin PR, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012; 64:2677–2686.
12. Waldmann TA, Wochner RD, Strober W. The role of the gastrointestinal tract in plasma protein metabolism Studies with 51Cr-albumin. Am J Med. 1969; 46:275–285.
13. MoK CC, Ying KY, Mak A, To CH, Szeto ML. Outcome of protein-losing gastroenteropathy in systemic lupus erythematosus treated with prednisolone and azathioprine. Rheumatology (Oxford). 2006; 45:425–429.

14. Yazici Y, Erkan D, Levine DM, Parker TS, Lockshin MD. Protein-losing enteropathy in systemic lupus erythematosus: report of a severe, persistent case and review of pathophysiology. Lupus. 2002; 11:119–123.

15. Chiu NT, Lee BF, Hwang SJ, Chang JM, Liu GC, Yu HS. Protein-losing enteropathy: diagnosis with (99m)Tc-labeled human serum albumin scintigraphy. Radiology. 2001; 219:86–90.

16. Chen Z, Li MT, Xu D, Yang H, Li J, Zhao JL. Protein-Losing Enteropathy in Systemic Lupus Erytematosus: 12 Years Experience from a Chinese Academic Center. PLoS One. 2014; 9:e114684.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download