Abstract
Radiofrequency ablation (RFA), a local ablative modality, is gaining acceptance for the treatment of liver malignancies. Despite a relatively low complication rate, tumor seeding resulting from RFA in hepatocellular carcinoma (HCC) treatment can occur. A 44-year-old woman was diagnosed with HCC. Spiral computed tomography (CT) revealed a 2.3 × 2.0-cm mass in the S5 segment, which was treated with RFA on May, 2005. Follow-up imaging, performed at 6-month intervals after RFA, showed complete tumor necrosis. In October 2009, CT revealed a heterogeneous mass, 5.7 cm in diameter, in the right ovary. Since the lesion was limited to the right ovary without evidence of spread, bilateral salpingo-oophorectomy was performed. Histopathology indicated that the metastatic spread from the HCC to the ovary was positive for hepatocyte-specific antigen on immunohistochemistry. The ovary is a rare site for HCC metastasis. Moreover, needle tract implantation of HCC in the ovary is very rare.
Go to : 
REFERENCES
1.McGahan JP., Browning PD., Brock JM., Tesluk H. Hepatic ablation using radiofrequency electrocautery. Invest Radiol. 1990. 25:267–70.

2.Rossi S., Fornari F., Pathies C., Buscarini L. Thermal lesions induced by 480 KHz localized current field in guinea pig and pig liver. Tumori. 1990. 76:54–7.

3.Llovet JM., Vilana R., Brú C., Bianchi L., Salmeron JM., Boix L, et al. Increased risk of tumor seeding after percutaneous radiofrequency ablation for single hepatocellular carcinoma. Hepatology. 2001. 33:1124–9.

4.Stigliano R., Marelli L., Yu D., Davies N., Patch D., Burroughs AK. Seeding following percutaneous diagnostic and therapeutic approaches for hepatocellular carcinoma. What is the risk and the outcome? Seeding risk for percutaneous approach of HCC. Cancer Treat Rev. 2007. 33:437–47.
5.Livraghi T., Goldberg SN., Lazzaroni S., Meloni F., Solbiati L., Gazelle GS. Small hepatocellular carcinoma: treatment with radio-frequency ablation versus ethanol injection. Radiology. 1999. 210:655–61.

6.Mulier S., Mulier P., Ni Y., Miao Y., Dupas B., Marchal G, et al. Complications of radiofrequency coagulation of liver tumours. Br J Surg. 2002. 89:1206–22.

7.Livraghi T., Solbiati L., Meloni MF., Gazelle GS., Halpern EF., Goldberg SN. Treatment of focal liver tumors with percutaneous radio-frequency ablation: complications encountered in a multi-center study. Radiology. 2003. 226:441–51.

8.De Baère T., Risse O., Kuoch V., Dromain C., Sengel C., Smayra T, et al. Adverse events during radiofrequency treatment of 582 hepatic tumors. AJR Am J Roentgenol. 2003. 181:695–700.

9.Jaskolka JD., Asch MR., Kachura JR., Ho Cs., Ossip M., Wong F, et al. Needle tract seeding after radiofrequency ablation of hepatic tumors. J Vasc Interv Radiol. 2005. 16:485–91.

10.Chen MH., Wei Y., Yan K., Gao W., Dai Y., Huo L, et al. Treatment strategy to optimize radiofrequency ablation for liver malignancies. J Vasc Interv Radiol. 2006. 17:671–83.

11.Shirato K., Morimoto M., Tomita N., Kokawa A., Sugi, ori K., Saito T, et al. Hepatocellular carcinoma: a case of extrahepatic seeding after percutaneous radiofrequency ablation using an expandable needle electrode. Hepatogastroenterology. 2002. 49:897–9.
12.YW C., SJ P., HY J., JY K., Lee JI., MJ K, et al. A case of needle tract seeding of hepatocellular carcinoma after radiofrequency ablation. Inje Med J. 2006. 27:127–32.
13.Markenson D. The treatment of children exposed to pathogens linked to bioterrorism. Infect Dis Clin North Am. 2005. 19:731–45.

14.Lencioni RA., Allgaier HP., Cioni D., Olschewski M., Deibert P., Crocetti L, et al. Small hepatocellular carcinoma in cirrhosis: randomized comparison of radio-frequency thermal ablation versus percutaneous ethanol injection. Radiology. 2003. 228:235–40.

Go to : 
 | Fig. 1.(A) Abdominopelvic computed tomography (CT) shows a low- density 2.3 cm mass (before radiofrequency ablation (RFA). (B) low-density lesion on the portal phase in S5 of the liver (post RFA). (C) Abdominopelvic CT reveals a 5.7 cm heterogeneous mass. (D) T2-weighted magnetic resonance image shows a high signal intensity mass in right ovary. |
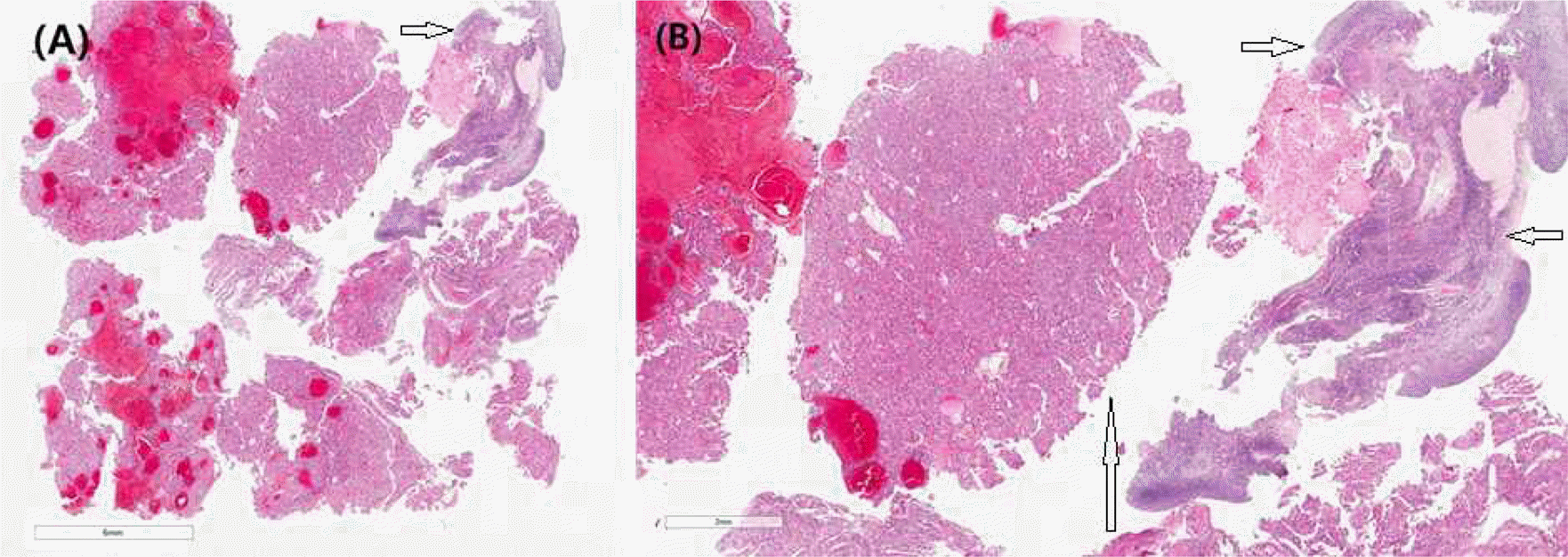 | Fig. 1.(A) Tissue from ovarian mass (Hematoxylin and Eosin Staining (H-&E) stain, x2). The ovarian mass compose of fragmented ovarian cortex, and tumor mass with hemorrhage. (B) Mid-magnification (H&-E stain, x20). The ovarian mass is of hepatocellular carcinoma with hemorrhage. (Long arrow: HCC, short arrow: Ovary) |
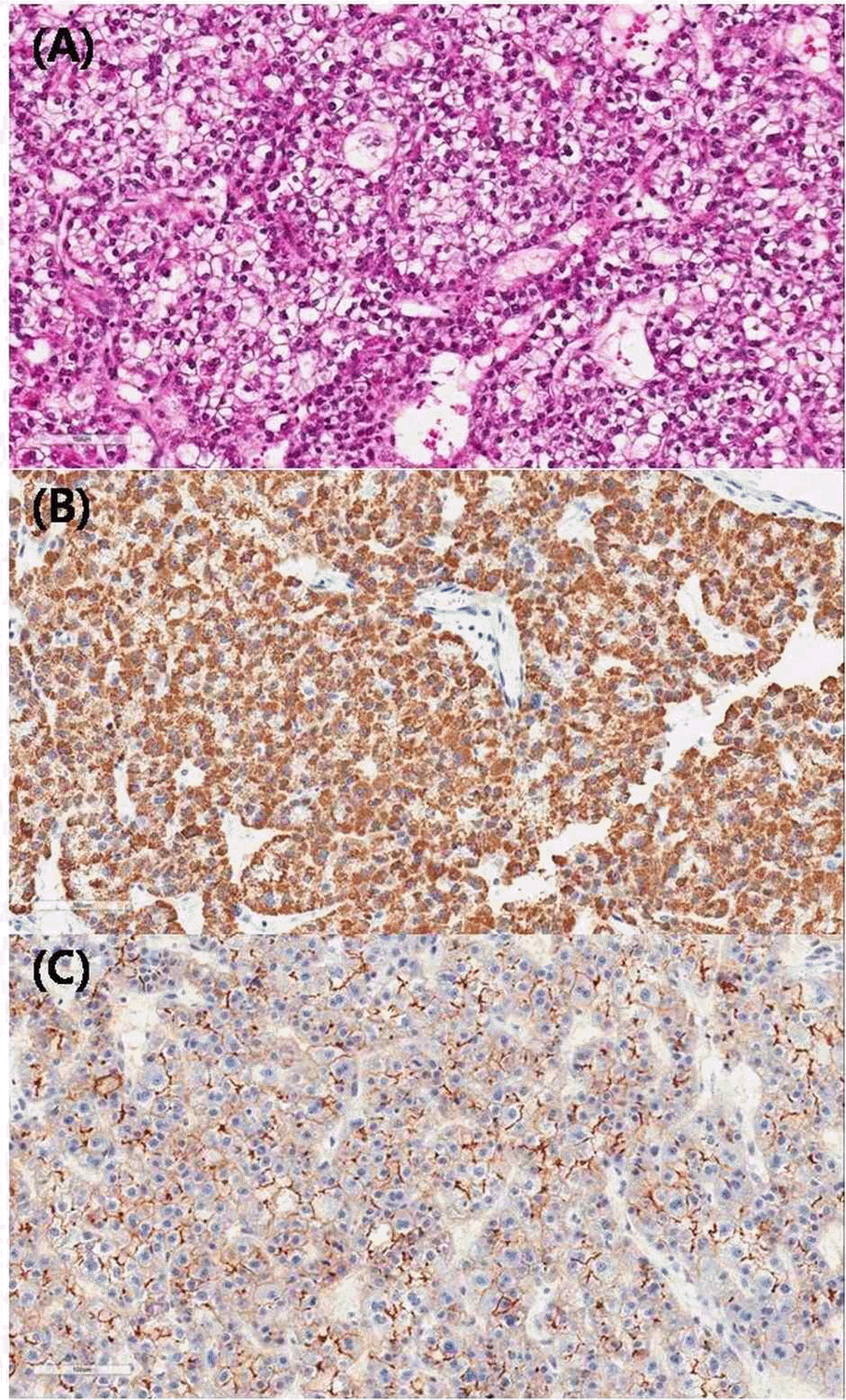 | Fig. 3.Higher magnification (A, H&-E; B, hepatocyte specific antigen immunohistochemistry (HSA); C, Carcinoembryonic Antigen immunohistochemistry (CEA), x200). (A) The hepatocellular carcinoma metastatic to vary shows thick trabecular and occasionally pseudo glandular architecture, clear cell type and nuclear grade 2/3 (common/worst). (B) Representing clear HAS (+) and (C) bile canalicular type of CEA. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download