Abstract
Stress induced cardiomyopathy is a disease that shows a dysfunction of the ventricle, but it can be rapidly reversible. It often occurs in older women primarily who suffers from emotional or physical stress. There are some case reports about postpartum stress induced cardiomyopathy. Most of the patients are recovered naturally within days to weeks. We report a case of a 37 years-old woman, who had experienced postpartum stress induced cardiomyopathy 8 years ago, revisited hospital because of cardiomyopathy after secondary delivery. Herein we report a rare case of recurrent stress induced cardiomyopathy after secondary normal vaginal delivery.
Stress induced cardiomyopathy (so called Tako-Tsubo cardiomyopathy or ampulla cardiomyopathy) is a disease that shows a dysfunction of the ventricle, but it can be rapidly reversible in a period by 4 to 8 weeks.1 It often occurs in older women primarily who suffers from emotional or physical stress.2 There are some case reports about postpartum stress induced cardiomyopathy in young women after spinal anesthesia, general anesthesia during Cesarean section.3 However postpartum stress induced cardiomyopathy after normal vaginal delivery is rarely reported and it is known that the recurrence rate is low. We report a rare case of recurrent stress induced cardiomyopathy after secondary normal vaginal delivery.
A 37-year-old woman has an admission history of ventricular fibrillation and stress induced cardiomyopathy related with the postpartum period, on 10 days after first normal vaginal delivery on September 22th 2005. At that time she arrived ER with ventricular fibrillation (Fig. 1A) and the ventricular fibrillation was conversed to regular sinus rhythm after defibrillation (Fig. 1B). After resuscitation, the ECG revealed sinus tachycardia and ST-segment elevation in the II, III, aVF leads and V1–V6 leads. Echocardiography was performed at admission day, and its findings were decreased ejection fraction to 38.9% and regional systolic dysfunction of the left ventricular walls with hypokinesis of the mid-apical segments and hyperkinesis of the basal segments. In coronary angiography, there were no stenotic lesions, but vasospasm was observed on left anterior descending by ergonovine injection without symptom and ECG change. A follow up echocardiography performed 2 weeks after admission showed improvement of the wall motion abnormalities.
Eight years later, she was pregnant with second child. She had no hypertension, diabetes mellitus, arrhythmia, history of syncope and medical problems except for admission history of 2005. She also had no smoking, drinking history and familial history. And she has been performed ECG and echocardiography follow up in concern about postpartum cardiomyopathy before delivery, but there was no abnormal findings. Ejection fraction was 60.1% and there was no regional wall motion abnormality (Fig. 2 A–C). On the 12 days after second normal vaginal delivery, she admitted to emergency room (ER), because of chest discomfort lasting 5 minutes, which started from date of visit and repeated twice. The echocardiography revealed findings were decreased ejection fraction to 50.2%, global hypokinesia from mid-ventricle to apex in LV and minimal pericardial effusion in the side of right ventricle (Fig. 2D–F).
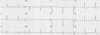
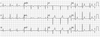
In this admission to our hospital, her blood pressure was 100/60 mmHg, heart rate was 60 bpm, body temperature was 36.1 ℃, respiratory rate was 20 /minutes. A chest radiograph showed no cardiomegaly and no pulmonary edema. The ECG showed ST-segment depression in the I, II, aVL, aVF, V3–6 leads (Fig. 3). In laboratory findings, complete blood count test were; white blood cells 6,100 cells/µL, hemoglobin 13.1 g/dL, hematocrit 39.2%, and platelets 164,000 cells/µL. A baseline cardiac enzymes were normal; troponin-T 0.076 ng/mL and CK-MB 2.03 ng/mL, but after 4 hours later it increased up to troponin-T 0.888 ng/mL and CK-MB 46.33 ng/mL. The serum brain natriuretic peptide was 185.7 pg/mL. Coronary angiography was not performed. After 2 days later, cardiac enzymes decreased to troponin-T 0.631 ng/mL and CK-MB 6.77 ng/mL, the ECG normalized (Fig. 4) and echocardiographic findings showed normal LV systolic function and normal LV regional wall motion. She had not appealed chest pain and the other symptoms after admission. She discharged 3 days after admission.
Stress induced cardiomyopathy is characterized by transient left ventricular systolic dysfunction, in the absence of obstructive coronary disease. The prevalence is unclear but number of case report of the stress induced cardiomyopathy is constantly increasing. It occurs typically in older women. Most patients with stress induced cardiomyopathy are postmenopausal women with a mean age of 58 to 77 years. Less than 3% of patients are younger than 50 years.4
The pathophysiology of stress induced cardiomyopathy remains unclear but the catecholamines are thought to play an important role by coronary vasospasm, dysfunction of microvessels, outflow tract obstruction, and direct effects of catecholamine on the cardiomyocytes.4
Stress induced cardiomyopathy often occurs in older women primarily who suffers from emotional or somatic stressors.2 The cause of postpartum stress-induced cardiomyopathy is similar. Physiologic or emotional stress associated with delivery like preeclamsia, caesarean delivery, and pain lead to catecholamine surge and this precipitates into postpartum stress-induced cardiomyopathy.5
The clinical presentation in most patients is indistinguishable from an acute coronary syndrome. Chest pain and dyspnea were reported most common symptoms. Patients can present with ST segment elevation, ST segment depression like our case, T wave inversion, non-specific ECG changes, or even a normal ECG.6 ST-elevation was observed in 81.6% patients, usually on precordial leads, and T wave abnormality was observed in 64.3% patients. The serum level of cardiac enzymes was elevated in more than 70% of patients.4
A number of diagnostic criteria are proposed, but the Mayo Clinic criteria have been internationally used. The criteria include (1) Transient hypokinesis, akinesis, or dyskinesis in the left ventricular mid segments with or without apical involvement; regional wall motion abnormalities that extend beyond a single epicardial vascular distribution; and frequently, but not always, a stressful trigger. (2) The absence of obstructive coronary disease or angiographic evidence of acute plaque rupture. (3) New ECG abnormalities (ST-segment elevation and/or T-wave inversion) or modest elevation in cardiac troponin. (4) The absence of pheochromocytoma and myocarditis.1
In our case, a 37-year-old woman visited hospital due to recurrent postpartum stress cardiomyopathy occurring about 10–12 days after delivery. Her diagnosis was postpartum stress induced cardiomyopathy, but we should think peripartum cardiomyopathy as differential diagnosis at same time.
Peripartum cardiomyopathy is a potentially life-threatening heart disease associated with pregnancy. It is an idiopathic cardiomyopathy presenting with heart failure secondary to left ventricular systolic dysfunction towards the end of pregnancy or in the months following delivery, where no other cause of heart failure is found. It is a diagnosis of exclusion. The left ventricle may not be dilated but the ejection fraction is nearly always reduced below 45.7
In our patient's case, the definition of peripartum cardiomyopathy is satisfied at first event and second event. But postpartum stress induced cardiomyopathy is more suitable diagnosis for her. Because at first event, her echocardiographic and ventriculographic findings showed regional systolic dysfunction of the left ventricular walls with hypokinesis of the mid-apical segments and hyperkinesis of the basal walls, a typical finding of stress induced cardiomyopathy which reported in above 85% of all patients described in the literature and so called Tako-Tsubo.8 There is similar reported case that a women's echocardiography showed dilatation of the left ventricle with apical ballooning and hyperkinesis of the basal segments after delivery and she diagnosed Tako-Tsubo cardiomyopathy.9 In most cases, stress induced cardiomyopathy is rapidly reversible. But the mortality has been reported about 1%, mostly associated with congestive heart failure and pulmonary edema.4 And the mortality rate of postpartum stress-induced cardiomyopathy is also estimated at 1%.10
This case presents interesting and many unique aspects.
The recurrence rate of stress induced syndrome is below 10%.1 Although the recurrence rate is low, her event was recurred after delivery. There are some case reports and reviews about postpartum stress induced cardiomyopathy after spinal anesthesia, general anesthesia during Cesarean section. But it is rarely reported about stress induced cardiomyopathy after normal vaginal delivery, not by Cesarean section. In previous cases associated with caesarean delivery, intravenous ergometrine or treatment of bradycardia and hypotension after spinal anesthesia with ephedrine and atropine therapy, were considered possible precipitating factors.3 Caesarean delivery itself is characterised by increased sympathetic tone, so endogenous and exogenous stresses were also considered to be precipitating factors of this cardiomyopathy.9 She probably would have been stressed emotionally and physically due to pregnancy. We think these factors might triggered her event.
Stress induced cardiomyopathy is believed recently as benign form of reversible cardiomyopathy, and the prognosis is generally favorable, but our patient's first attack was life threatening.4 There are similar other reports including two patients died suddenly of ventricular fibrillation and torsade de pointes after recovery of LV function.11 And serious clinical presentations such as cardiogenic shock and ventricular fibrillation were 4.2% and 1.5% on 2006.4 At her second event, the echocardiography revealed global hypokinesia from mid-ventricle to apex in LV. It is not typical finding of apical ballooning on echocardiography. But other variants are also common, including basal or midmyocardial akinesia with preserved apical function. Mayo clinic diagnostic criteria include regional wall motion abnormalities that beyond a single epicardial vascular distribution and it was met in second case.1
Our case has certain limitation. She did not have coronary angiography and cardiac MRI. Because her symptoms and examinations were improved, she was clearly diagnosed postpartum stress induced cardiomyopathy before 8 years ago and did not want to have coronary angiography and cardiac MRI. Coronary angiography is usually necessary to differentiate it from an acute coronary syndrome with plaque rupture, thrombus, or embolism. And cardiac MRI is helpful in differentiating stress induced cardiomyopathy from acute myocardial infarction and myocarditis.
Herein the case report about recurrent postpartum cardiomyopathy that have interesting and many unique aspects. Although recurrence rate is low in the literature, do not pregnant again is important to avoid pregnancy again is important for her to prevent recurrence.
Figures and Tables
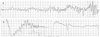 | Fig. 1ECG Electrocardiogram in the EMS ambulance after first normal vaginal delivery on September 22th 2005. The ventricular fibrillation (A) was conversed to regular sinus rhythm after defibrillation (B). EMS: emergency medical service. |
 | Fig. 2Echocardiography was showed that increased dimension and volume of LV after second normal vaginal delivery.A: LVEDD (LV end diastolic dimension) of LV basal level at parasternal view (M-mode) was 45mm before second normal vaginal delivery.
B: LVEDD (LV end diastolic dimension) of LV mid-portion at parasternal view (M-mode) was 45mm before second normal vaginal delivery.
C: LVEDV (LV end diastolic volume) was 82.7ml at apical 4 chamber view before second normal vaginal delivery.
D: LVEDD (LV end diastolic dimension) of LV basal level at parasternal view (M-mode) was 50mm on the 12 days after second normal vaginal delivery.
E: LVEDD (LV end diastolic dimension) of LV mid-portion at parasternal view (M-mode) was 53mm on the 12 days after second normal vaginal delivery.
F: LVEDV (LV end diastolic volume) was 107.4ml at apical 4 chamber view on the 12 days after second normal vaginal delivery.
|
References
1. Prasad A, Lerman A, Rihal CS. Apical ballooning syndrome (Tako-Tsubo or stress cardiomyopathy): a mimic of acute myocardial infarction. Am Heart J. 2008; 155:408–417.

2. Wittstein IS, Thiemann DR, Lima JA, Baughman KL, Schulman SP, Gerstenblith G, et al. Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med. 2005; 352:539–548.

3. Jo YY, Park S, Choi YS. Extracorporeal membrane oxygenation in a patient with stress-induced cardiomyopathy after caesarean section. Anaesth Intensive Care. 2011; 39:954–957.

4. Gianni M, Dentali F, Grandi AM, Sumner G, Hiralal R, Lonn E. Apical ballooning syndrome or takotsubo cardiomyopathy: a systematic review. Eur Heart J. 2006; 27:1523–1529.

5. Jeong H, Lee S, Jeong C, Lee J, Jeong S, Chung S, et al. Inverted Takotsubo-Like Left Ventricular Dysfunction with Pulmonary Oedema Developed after Caesarean Delivery Complicated by Massive Haemorrhage in a Severe Preeclamptic Parturient with a Prolonged Painful Labour. Case Rep Anesthesiol. 2011; 2011:164720.

6. Ghadri JR, Ruschitzka F, Lüscher TF, Templin C. Takotsubo cardiomyopathy: still much more to learn. Heart. 2014; 100:1804–1812.

7. Caforio AL1, Pankuweit S, Arbustini E, Basso C, Gimeno-Blanes J, Felix SB, et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2013; 34:2636–2648.

8. Redfors B, Shao Y, Omerovic E. Stress-induced cardiomyopathy (Takotsubo)--broken heart and mind? Vasc Health Risk Manag. 2013; 9:149–154.
9. Citro R, Pascotto M, Provenza G, Gregorio G, Bossone E. Transient left ventricular ballooning (tako-tsubo cardiomyopathy) soon after intravenous ergonovine injection following caesarean delivery. Int J Cardiol. 2010; 138:e31–e34.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download